

After (Mg,Fe)SiO3 perovskite, (Mg,Fe)O is likely the next most abundant mineral in the lower mantle. The crystal chemistry of (Mg,Fe)O, particularly with respect to Fe3+, is important for understanding properties of the lower mantle such as electrical conductivity. We have already measured the extent of Fe3+ solubility in Fe0.2Mg0.8O at 1 bar as a function of temperature and fO2, (see Annual Report 1993), and developed a technique for determining accurate values for Fe3+/Σ Fe in (Fe,Mg)O using Mössbauer spectroscopy and X-ray diffraction. We found that the Fe3+ composition is a strong function of fO2, where large amounts of Fe3+ (Fe3+/Σ Fe > 10%) can be incorporated in the structure at high fO2.
To study the solubility of Fe3+ in (Mg,Fe)O at high P,T,
two samples of Fe0.2Mg0.8O were prepared, one with
a high Fe3+ content and one containing minimum Fe3+.
Both were run in a multianvil press at 18 GPa and 1000 °C at two different
oxygen fugacities: a) in equilibrium with Fe (reduced); and b) in equilibrium
with a Re-ReO2 buffer (oxidised). In all high pressure experiments,
the amount of Fe3+ found in the quenched phases using both Mössbauer
spectroscopy and X-ray diffraction was extremely small, including in the
phase starting with a high Fe3+ content and run under oxidising
conditions. This implies that the solubility of Fe3+ becomes
very low in Fe0.2Mg0.8O at high pressure, regardless
of fO2. The difference in solubility of Fe3+
between high and low pressure is shown in Fig. 3.3-8, illustrating the
dramatic decrease in Fe3+ solubility at high pressure. One explanation
for this behaviour is the presence of high-pressure phase transitions in
both Fe3O4 and MgFe2O4 which
stabilises oxygen in the spinel phase at high pressure, hence producing
a more stoichiometric (Mg,Fe)O composition.
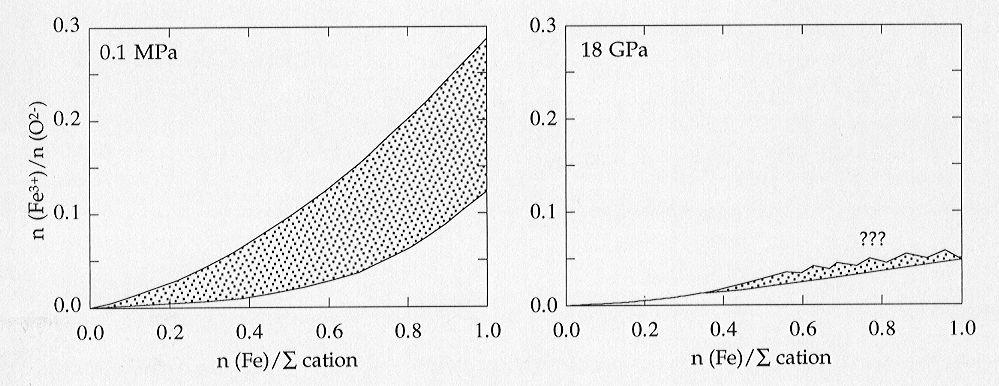 |
Fig. 3.3-8: Solubility of Fe3+ in (Mg,Fe)O at 1000 °C as a function of composition at atmospheric pressure (left) and at 18 GPa (right). Phase boundaries in the left figure and data for FexO in the right figure are derived from data in the literature. No data are available yet for iron-rich (Mg,Fe)O at 18 GPa and high oxygen fugacity. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page