

Boron is present in natural granitic magmas and associated fluid phases at trace to minor concentration levels. The presence of boron in magmatic systems is normally revealed by the crystallization of borosilicate minerals like tourmaline or dumortierite and by the composition of fluid inclusions in some pegmatites. The effect of boron on the physical and chemical properties of natural magmas and synthetic silicate melts is of great importance. In particular, the minor- and trace-element chemistry of highly differentiated magmas is profoundly affected by the presence of boron. On the basis of measured values of boron contents in tourmaline granites, pegmatites and in obsidian glasses, the maximum B2O3 concentration in magmas has been placed at 1 wt%. However, silicate melts with B2O3 concentrations higher than 1 wt% may well exist in nature and may be present during the very late stages of igneous fractionation. Therefore, we conducted an experimental study of the composition-dependence of water solubility and speciation in the system NaAlSi3O8-KAlSi3O8-SiO2-B2O3-H2O at 800 °C and 1-3 kbar.
The experimental procedure involved 1 atm dry oxide fusion, hydrothermal
saturation, isobaric rapid quench, Karl-Fischer titration analysis for
water and microscopic investigation of homogeneity and water speciation
using infrared absorption spectroscopy. The solubility of water in haplogranitic
melt at 2 kbars was found to increase from 5.9 wt% to 8.9 wt% upon addition
of 9 wt% B2O3. Boron strongly influences the speciation
of water in the melt by shifting the OH/H2O-equilibrium towards
higher contents of hydroxyl groups (Fig. 3.6-6). This observation cannot
be quantified without an evaluation of quench effects.
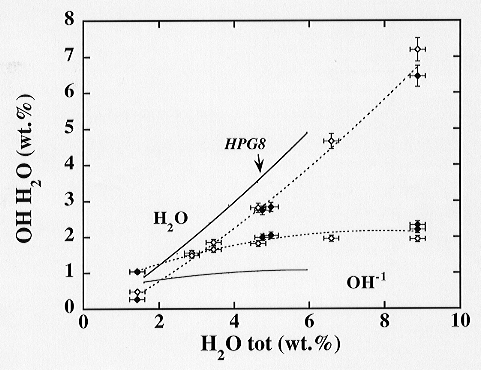 |
Fig. 3.6-6: Proportion of OH and H2O in haplogranitic glass containing 9 wt% B2O3 quenched from 800 °C and 2 kbars. Results for the boron-free system are shown for comparison (solid line). |
The water speciation analyzed in glasses records in part the state of
homogeneous equilibria that exists at the glass transition temperature.
Thus, to analyze the equilibrium speciation of water as a function of temperature
we require glass transition temperatures for our quenched run products.
Micropenetration viscometric experiments in the range of 109
to 1012 Pa s reveal a smooth decrease in the Tg from 900 °C
to 350 °C as a function of combined water and boron content of these
glasses (Fig. 3.6-7). In this figure, all the experiments are reported
at the same quench rate of 20 °C/min and therefore Tg is defined as
the temperature at which the viscosity reaches 109 Pa s. The
basis of the compositional variable in Fig. 3.6-7 is mole fraction OH +
mole fraction B2O3/6. We choose this basis for empirical
reasons; it results in the smoothest variation of Tg with water and boron
contents over the compositional range studied.
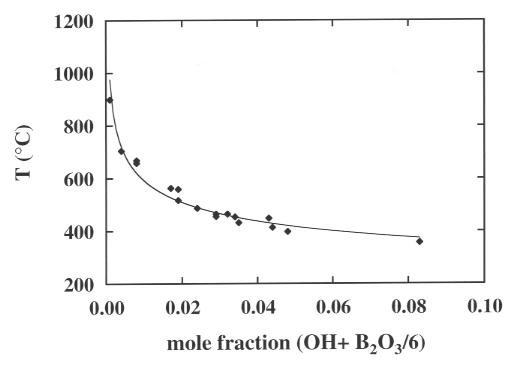 |
Fig. 3.6-7: Glass transformation temperature as a function of OH + B2O3/6 content in haplogranitic melt. |
After successful parameterization of the Tg variations in this system
we recalculated the proportions of species frozen at Tg to a constant temperature
using the temperature dependence of water speciation as determined previously
for glasses of hydrous feldspar composition. The enthalpy for that reaction,
36 (± 5) KJ/mol, is similar to that recently
obtained using in situ spectroscopy on a haplogranitic melt (34
KJ/mol) implying a melt composition invariance of the enthalpy. Figure
3.6-8 illustrates the isothermal (corrected to T = 1000 °C) concentrations
of OH and molecular H2O in a boron-rich melt. Corrected for
the temperature dependence, the compositional influence of boron on the
proportion of hydroxyl and molecular water is now seen to be very large.
This extraordinary effect of boron on the speciation of water in granitic
melts will significantly influence the physicochemical behaviour of boron-rich
late stage granitic magmas.
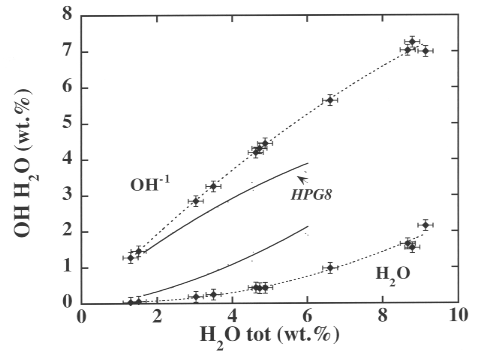 |
| Fig. 3.6-8: Proportion of OH and H2O in haplogranitic melt with 9 wt% B2O3 at 2 kbars and 1000 °C. Results for the boron-free system are shown for comparison (solid line). |
Pressure has no measurable influence on the speciation of water in haplogranitic melts between 1 and 3 kbars. This implies that the speciation reaction has a relatively small ΔV of reaction, consistent with the constant value of the partial molar volume of water in sodium silicate melts reported in literature. If generalisable, this observation will greatly simplify the task of estimating the volumetric properties of hydrous melts.
Very recent results involving in situ IR spectroscopy of a hydrous granitic melt indicate that a component of elastic displacive equilibria below Tg may be required to complete our picture of the temperature dependence of the speciation of water in melts. The enthalpy of the liquid state reaction of water speciation from in situ studies is identical to that provided in the present study. This agreement between the results of the present study and those of the in situ measurements imply that the major influence of temperature on melt speciation does indeed freeze in at the glass transition, but that minor (low activation energy) adjustments in water speciation do occur below the glass transition. Further comparisons of quenched versus in situ studies of melt and glass speciation should help to elucidate the nature of melt relaxation during cooling.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page