

Knowledge of the stability, speciation and solubility of hydroxyl in mantle minerals is required for understanding the budget and evolution of volatiles in the Earth. Anhydrous mantle minerals such as garnets contain only trace amounts of OH (less than a few hundred parts per million H2O by weight) yet this amount is significant in contributing to the total water budget in the Earth. Infrared absorption spectroscopy is a highly sensitive probe of OH concentration and of local structural environments and is therefore ideal for the characterization of hydroxyl in anhydrous minerals. Pure pyrope garnet was chosen in this study as a model for water solubility in mantle garnets.
Annealing experiments on single crystals of natural pyrope (Dora Maira Massif, Western Alps) under water saturated conditions were performed in a piston-cylinder apparatus and infrared spectra were collected on the recovered specimens to study speciation and solubility of water. Pyrope single crystals surrounded by pyrope powder together with pure water were sealed in platinum capsules and annealed at temperatures of 800 °C, 1000 °C and 1200 °C and pressures between 15 kbar and 30 kbar for durations ranging from 4 hours to 6 days. Oxygen fugacity was buffered by the Ni-NiO equilibrium. Quantitative analyses of OH concentrations were performed on doubly polished pyrope crystals recovered from the annealing experiments.
Infrared spectra in the near-IR region (Fig. 3.6-9) show that at least twice as much hydroxyl (>100 ppm H2O) as compared to that in the original samples can be introduced into the pyrope structure. Experiments at isothermal and isobaric conditions show that solubility of hydroxyl systematically increases with increasing pressure and decrease with increasing temperature. IR spectra also show systematics in speciations of hydroxyl bands. Intensity of the higher frequency multiple OH bands (3630 - 3660 cm-1) is apparently inversely proportional to that of the lower frequency OH band (3602 cm-1; Fig. 3.6-9). Experiments performed at various durations further suggest that the kinetics of water incorporation in pyrope is rather slow even at 1000 °C.
The speciation of hydroxyl was studied at high temperatures up to 1000
°C and ambient pressure with a heating stage (Fig. 3.6-10). The higher
frequency multiple bands showed stronger frequency shifts (-0.04 cm-1/°C)
with temperature than the lower frequency band (-0.02 cm-1/°C).
This difference suggests that they originate in sites with different expansivities.
All observed OH bands show negative temperature shifts which indicate weak
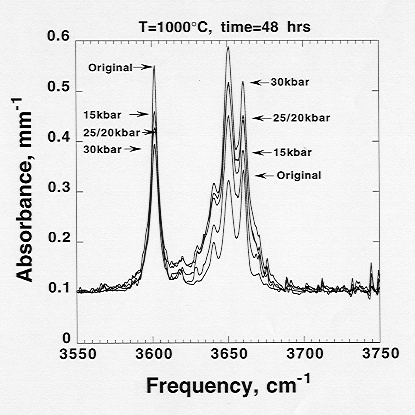 |
Fig. 3.6-9: Near infrared spectra showing the effect of annealing pressure on solubility of water in pyrope. The area under a spectral profile is proportional to the water concentration. Note: the total area over the whole range increases with annealing pressure while the area under the two groups of bands are inversely proportional. |
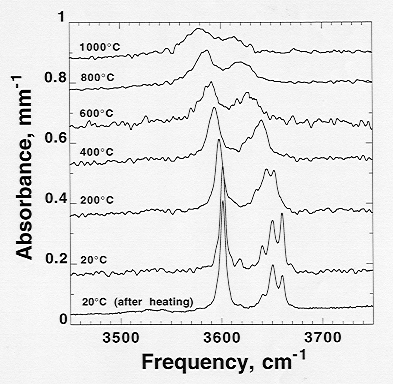 |
Fig. 3.6-10: Near infrared spectra showing effect of temperature on vibrational frequencies of hydroxyl bands in pyrope. The frequencies linearly decrease with increasing temperature. |
or negligible hydrogen bonding. Frequency shifts are fully reversible for temperatures up to 1000 °C within the experimental duration of less than one hour.
Water solubility in pyrope observed in our current annealing experiments indicates that pyrope, which is considered among the driest mantle phases, can incorporate about as much water as is present in the upper mantle.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page