

Despite the relatively large sample volume in multianvil experiments it is difficult to apply usual buffering methods such as a double capsule technique to control oxygen fugacity. It is therefore important to understand the constraints on oxygen fugacity in experiments which are performed to simulate mantle conditions.
Experiments with mantle phases have been performed to investigate the reliability of common methods which are used to impose and/or deduce redox conditions:
- Relative oxygen fugacities can be inferred from experiments performed at fixed conditions of pressure, temperature, time and composition (P, T, t, x) but using different capsule materials, assuming that the positions of buffer curves relative to one another are preserved at high pressure.
- The variation of starting material composition (e.g. oxygen content, reducing components) can be used to impose different redox states while P, T, t conditions of the experiments remain fixed.
- The fO2 values (relative to a metal-metal oxide buffer) can be estimated for experiments containing a metal M (e.g. Fe) and a corresponding MOx-bearing phase (e.g. magnesio-wüstite, mw) according to the relation: log fO2 (rel. to IW) = 2* log (aFeOmw / aFemet). This simple calculation assumes ideal activities of both components.
The investigation of the samples allows conclusions to be made about (a) the buffering capacity of the capsule material or the sample composition and (b) the reliability of fO2 values calculated from the phase relations of the sample:
- The Fe3+ content of different mantle minerals (e.g. majorite, perovskite) at experimental conditions were directly measured with Mössbauer spectroscopy, and used to infer the relative redox states provided by different capsule materials. Results suggested that different capsule materials indeed impose different oxygen fugacities, thus indicating that the capsule material may provide sufficient buffering capacity for relatively short run times.
- A systematic variation of Si and O contents in the starting material of partitioning experiments between liquid metal and magnesiowüstite at high pressures (9 and 18 GPa) and high temperature (2200°) was used to impose different oxygen fugacities. The fO2 values estimated using the above equation show a variation of 4 log bar units. The results confirm that changes of the starting material at given P, T conditions can indeed establish different oxygen fugacity levels.
- A comparison of fO2 data calculated assuming that Fe in Fe-Ni-liquids and FeO in magnesiowüstite display ideal mixing, (i.e. the activity coefficients equal 1) with fO2 constraints employing activity coefficients given in the literature shows that the two data sets agree within 0.5 log bar units. This result suggests that such a calculation can be a valuable tool for obtaining fO2 estimates even if the respective activity relations at high pressures and temperatures are not known.
- Further information on the redox conditions in multianvil experiments is obtained by a temperature-dependent set of magnesiowüstite - liquid metal equilibria. Figure 3.2-5 shows the calculated oxygen fugacities as a function of temperature.
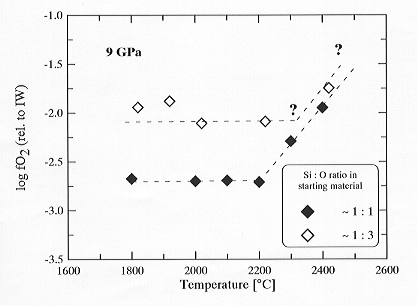 |
| Fig. 3.2-5: Calculated log fO2 values for metal-magnesiowüstite assemblages as a function of temperature. The oxygen fugacity is internally buffered below a 'critical' temperature above which it rapidly increases. |
Below a certain 'critical' temperature the calculated oxygen fugacity is essentially constant while above it rapidly increases with increasing temperature. The increase appears to be nearly linear with a value ˜ 0.4 log bar units per 100°C. The absolute value of the `critical´ temperature (˜ 2200°C for the investigated composition at 9 GPa) may vary with experimental parameters. To further constrain the nature of these relations, corresponding investigations at different fO2 levels are under way (indicated by open symbols in the figure). Preliminary results indicate that the fO2 values show also a weak dependence on pressure such that with increasing pressure the redox conditions slightly increase. These investigations imply that the buffering capacity of the sample composition can be restricted to certain P, T, t conditions. As a consequence the results of high pressure experiments, particularly those as a function of temperature, may be interpreted erroneously if the influence of oxygen fugacity is not adequately considered.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page