

The presence of hydrogen-bearing materials in the deep Earth should have considerable influence on the pressures and temperatures of melt production, and on the nature of the melts produced. The hydrous phases known at near-surface conditions all become unstable at fairly shallow depths, thus the hydrous mineralogy must change. Among the hydrogen-bearing candidate minerals are the so-called "alphabet" phases, which are stable to high pressures and temperatures; however, most of them have Mg/Si ratios that are too high for them to occur in the Earth. The phase described here was synthesized at 13.5 GPa and 1100°C, but is stable to much higher pressure and temperature.
The starting material for synthesis was a mixture of lawsonite, zoisite and Al(OH)3, with the equivalent of 12% H2O contained in the mixture. The resulting material consists of stishovite, diaspore, and the phase of interest, which was first synthesized in 1978, but the X-ray techniques then available were too primitive for the complex pattern to be indexed, and solution of the structure was clearly impossible. The present X-ray diffractogram was collected on the high-resolution instrument at beamline BM16 of the European Synchrotron Radiation Facility in Grenoble. With the modern equipment, the pattern could be indexed without ambiguity and integrated intensities could be extracted from the multiphase pattern using the computer program GSAS. These data were then used to solve the crystal structure using the program SIRPOW. The structure was then refined using program GSAS. The material is monoclinic with space group P21/n and lattice parameters a=7.1144(1), b=4.3347(1), c=6.9527 and ß =98.40(1).
In Fig. 3.3-9a, which is an a-c view of the structure,
the fundamental building block, which consists of a chain of edge-shared
octahedra parallel to the a axis, is shown. Si and Al are completely
ordered over the octahedral sites. In Fig. 3.3-9b, which is a b-c
projection, the corner linking of these units to create open channels parallel
to a, is demonstrated. These channels provide space for the OH..O
hydrogen bond as indicated in the figure, but are too small for H2O
to be contained within them, thus all hydrogen occurs in the form of OH.
The larger sphere represents this OH group, and the smaller one the receptor
oxygen. These ions were identified from bond strength sums.
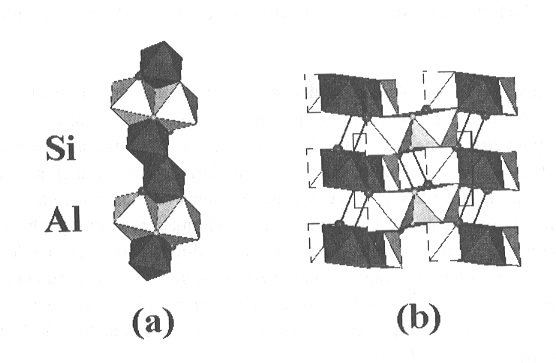 |
Fig. 3.3-9: Drawings of the crystal structure of AlSiO3OH. (a) An a-c view that shows the edge-shared octahedral strip extending parallel to the a axis. (b) A b-c projection showing the corner linking of these strips to form channels parallel to a. Silicon octahedra are drawn in a darker shade than those containing aluminum, the OH group is indicated by the larger sphere, and the receptor in the OH..O bond by the smaller sphere. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page