

The most common natural examples of mafic high-pressure minerals, like those found in the deep Earth, occur in melt veins within chondritic meteorites which result from shock metamorphism during impact events on the chondritic parent bodies. The mineral assemblages in these melt veins provide important information concerning the pressure and temperature conditions of melt-vein crystallization when combined with information from dynamic and static high-pressure experiments. As part of an ongoing study of high pressure minerals in shock veins (Annual Report 1995) we investigated the ACFER 040 L5-6 (S6) chondrite from the Algerian Sahara that we suspected of having experienced very high pressures. The sample is a breccia that contains a large number of black melt veins. The matrix of the melt-vein was examined by analytical transmission electron microscopy to better understand the conditions of melt vein crystallization during shock metamorphism.
The melt vein matrix consists of a mixture of equant pockets of amorphous material of approximately 2 µm (1.9 ± 0.4 µm) in size, surrounded by finer mineral grains and interstitial glass (Fig. 3.3-11A). Electron diffraction patterns collected from the pockets (Fig. 3.3-11A) confirm that they are amorphous, although in some cases there are tiny inclusions (40 to 250 nm) of crystalline material. EDS analyses of the amorphous pockets show that they have a composition very similar to that of the majorites that commonly occur in the matrix of shock-melt veins. The compositions of the amorphous pockets are quite distinct from that of the interstitial glass that occurs between the surrounding crystals (Fig. 3.3-11B), which contains a substantial amount of P2O5 as well as more CaO and less FeO than the amorphous pockets.
The predominant crystalline phase between the amorphous pockets (Fig. 3.3-11A, B) has a composition similar to enstatite (MgSiO3) with small amounts of Al2O3, Na2O and Cr2O3. Electron diffraction patterns from these crystals can be indexed as MgSiO3-ilmenite ( Fig. 3.3-11B), a high-pressure polymorph of enstatite. To be certain that we had the ilmenite structure, multiple electron diffraction patterns were collected from individual MgSiO3-ilmenite grains and indexed using d-spacings, angles and systematic absences. The zone axes were then plotted on a stereographic projection to show that the electron diffraction data fit the ilmenite structure in three dimensions. From this diffraction data we can rule out all other known polymorphs of MgSiO3-ilmenite including the very similar corundum structure (R-3c versus R-3 space groups). The MgSiO3-ilmenite grains have variable morphologies, with most forming prismatic crystals up to several microns long. Many grains contain tiny planar defects that are visible as small spots or platelets with local strain contrast (Fig. 3.3-11B). The second crystalline phase in the melt-vein matrix is ringwoodite (Fig. 3.3-11B), the spinel-structure polymorph of (Mg,Fe)2SiO4 olivine. Ringwoodite grains can be either equant in dimension or somewhat elongated, ranging in size from about 400 nm to about 1 µm. They have very high densities of stacking faults on {110} planes, with the faults commonly occurring as short (100 to 200 nm) segments. Intergrown ringwoodite and MgSiO3-ilmenite suggest that these two phases crystallized simultaneously.
The amorphous pockets in the matrix cannot be easily explained as a
quenched remnant of melt because of the non-random distribution of the
surrounding crystals. Homogeneous nucleation of crystals in a super-cooled
melt would not produce crystal-free pockets surrounded by crystals.
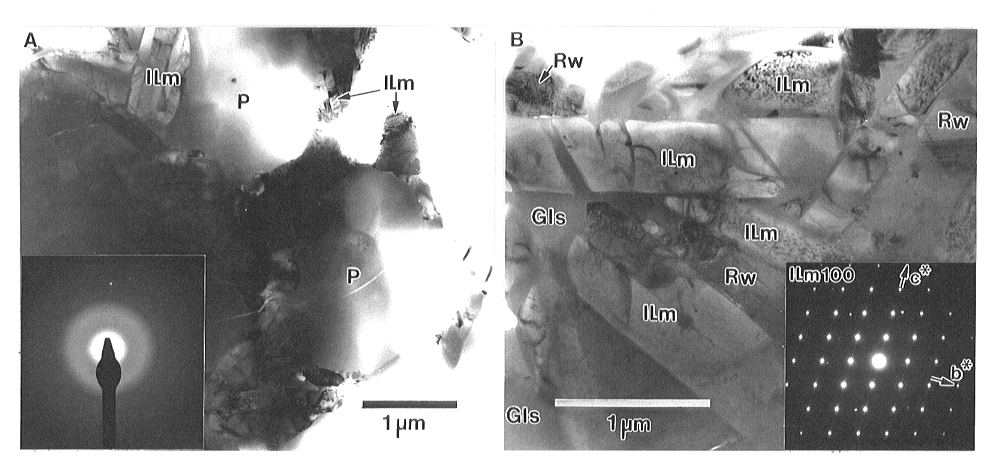 |
| Fig. 3.3-11: Bright-field TEM images of the melt vein matrix
showing
(A) amorphous pockets (P) surrounded by crystals of MgSiO3-ilmenite (Ilm) and ringwoodite. Electron diffraction from the pockets (A) demonstrates that they are amorphous. (B): Between the pockets, the matrix consists of prismatic grains of MgSiO3-ilmenite (Ilm) as well as ringwoodite (Rw) and interstitial glass (Gls). The [100] zone axis of MgSiO3-ilmenite is also shown. Many of the MgSiO3-ilmenite grains (B) have lattice defects that appear as dark spots in images and produce streaking along c* in diffraction patterns. |
The distinct difference in composition between the amorphous pockets and the interstitial glass provides further evidence against the pockets being remnants of quenched melt. A possible explanation is that the amorphous pockets represent crystalline material at high-pressure which became amorphous after pressure release. MgSiO3-perovskite, which has a composition similar to that of majorite, is a likely candidate to explain the amorphous pockets because it is extremely unstable at low pressure and is known to become amorphous at one atmosphere if heated to a temperature greater than 150°C or when crushed in a mortar and pestle at room temperature. The compositions of the amorphous grains are within the compositional range of MgSiO3-perovskites synthesized in chemically complex systems. We conclude that the amorphous pockets represent MgSiO3-perovskite that crystallized from the melt at high pressure but amorphized after pressure release. The post-shock waste heat that is produced during decompression of shocked materials can produce temperatures as high as 1750°C which would preclude the survival of crystalline (MgFe)SiO3-perovskite in shocked chondrites.
Crystallization of the melt vein appears to have involved three silicate phases as well as Fe-Ni metal and troilite. The distribution of minerals and glass pockets suggests a sequence of crystallization that started with nucleation and growth of MgSiO3-perovskite followed by the simultaneous nucleation and growth of MgSiO3-ilmenite and ringwoodite at high pressure and temperature. This was then followed by the crystallization of the metal-sulfide melt at ˜ 875°C. The assemblage consisting of MgSiO3-perovskite, MgSiO3-ilmenite, and ringwoodite is not predicted by the liquidus phase diagram for chondritic compositions. This may reflect the relatively high P2O5 content of the melt phase, which is known to strongly effect phase equilibria in Fe-bearing silicate melts. Another explanation is simply that the assemblage cannot be approximated by an equilibrium phase diagram because the crystallization was occurring while the pressure and temperature were rapidly decreasing during the release phase of shock metamorphism. As far as we know, this is the first natural occurrence of MgSiO3-ilmenite, which therefore represents the discovery of a new mineral species. Because of the extreme instability of MgSiO3-perovskite, it is very unlikely that we will ever find crystalline MgSiO3-perovskite in natural samples. The presence of MgSiO3-ilmenite and amorphized MgSiO3-perovskite represents a higher crystallization pressure and temperature than the more common majorite-bearing assemblages found in the melt veins of many other shocked chondrites.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page