

The incomplete occupation of d electron energy levels in d9 and high-spin d4 transition metal cations leads to anisotropic interactions between the d electrons and the crystal field of co-ordinating ligands. As a result the regular coordination geometry spontaneously distorts to stabilize the electronic configuration. This effect, well known as Jahn-Teller (JT) effect, is responsible for the particular stereochemistry of d4 and d9 transition-metal cations such as Mn3+. The influence of pressure on the type and degree of distortion has not previously been investigated systematically. Braunite, Mn2+Mn3+6O8SiO4, was chosen in order to study the pressure dependence of the polyhedral geometries of the JT distorted Mn3+O6 octahedra because: (1) three crystallographically independent Mn3+ sites allow the influence of the topology on the polyhedral compression to be studied; (2) the common edge-sharing by polyhedra leads us to expect that intra-polyhedral changes dominate compression mechanisms.
A single-crystal X-ray diffraction study was carried out at seven different pressures up to 7.7 GPa in order to determine the pressure-induced structural changes. In addition to the structure refinements, we determined the equation of state (EOS) at room temperature (=298 K) from accurately measured unit-cell volumes up to 9.2 GPa. Pressures were determined to a precision better than ±0.02 GPa from the unit-cell volumes of fluorite and quartz internal diffraction standards (see also Section 3.8a). The high-pressure study was carried out in one of our in-house-designed diamond-anvil cells and the measurements of the accurate unit-cell parameters were performed with the new Huber four-circle diffractometer (see Annual Report 1995).
A 3rd order Birch-Murnaghan EOS was fitted to the experimentally
determined P-V data set yielding:
| V0 = 1661.15 ± 0.08 Å3 | K0,298 = 180.7 ± 0.9 GPa | K' = 6.5 ± 0.3 |
| The pressure dependence of the lattice parameters was found to be non-linear indicating a small anisotropy for the structural compression: | ||
| a0 = 9.4262 ± 0.0004 Å | K(a)0 = 498.7 ± 3.5 GPa | K' = 19.7 ± 0.9 |
| c0 = 18.6964 ± 0.0006 Å | K(c)0 = 656.9 ± 5.7 GPa | K' = 15.7 ± 1.4 |
The large bulk modulus can be related to the structural topology: 2/3 and 1/2 of all Mn2+O8 and Mn3+O6 polyhedral edges, respectively, are edges shared with other polyhedra. Therefore, the structure appears to be unable to compress by mechanisms of inter-polyhedral tilting and polyhedral rotation as observed for softer materials. The compressional anisotropy can be related to the fact that the most compressible Mn-O bonds are aligned closer to the (001) plane than to the c-axis. Significant pressure-induced changes can be observed for all Mn-O bond distances for the Mn2+O8 and Mn3+O6 polyhedra. The Mn(1)O8 polyhedron was found to compress isotropically: the linear mean compressibility coefficients for the Mn(1)-O1 and Mn(1)-O2 bonds are the same within their esd's. However, anisotropic compressional behaviour was observed as the octahedral coordination of Mn3+ cations is expected to be distorted as a result of the JT effect.
Although the polyhedral geometry of all three crystallographically independent
Mn3+ sites, Mn(2), Mn(3), and Mn(4), shows the same type of
uniaxially elongated distortion, the compression of the individual octahedral
configurations was found to be very different (Fig. 3.3-12). The comparison
shows that the Mn3+O6 polyhedral compression is dependent
upon both the geometrical features of the polyhedron itself and the types
of, and the connectivity to, the neighbouring polyhedra. Due to the differences
in the bonding of the oxygen ligands to different types of other cations
(O1 and O2 are coordinated by 3 Mn3+ + 1 Mn2+ atoms,
whereas O3 is coordinated by 3 Mn3+ + 1 Si) the Mn-O3 bonds
are generally more elongated compared to the Mn-O1 and Mn-O2 bonds, which
leads to topologically-induced polyhedral elongation. This additional distortion
appears to be suppressed as pressure increases (see Mn(4)O6).
Differences in the connectivity, e.g. the cis-configuration of shared
edges within the Mn(3)O6 octahedra in contrast to the trans-configured
Mn(2)O6 and Mn(4)O6 octahedra, lead to topologically-induced
bond-angle distortions and consequently to different O···O
repulsions which are responsible for the strong compressional anisotropy
within the square-planar base of the Mn(3)O6 polyhedron.
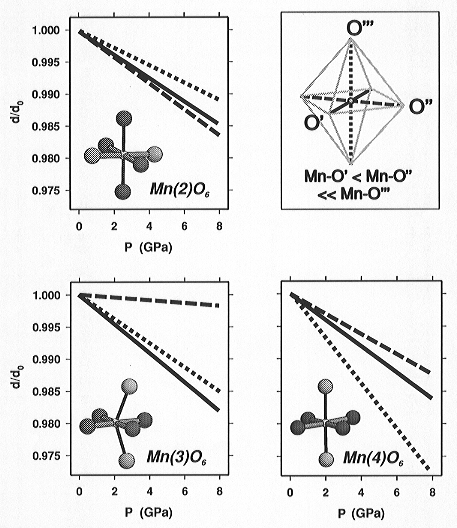 |
Fig. 3.3-12: Compression of the Mn3+O6 polyhedra in synthetic braunite up to 8 GPa. Line types differentiate the different bonds (solid line: shortest Mn-O bond length; dashed: intermediate; dotted: longest bond). O1 and O2 atoms: dark grey balls, O3: light grey. |
The high-pressure study on braunite shows that the structural topology appears to predetermine the behaviour of polyhedral compression. Therefore, the influence of pressure on the JT effect can only be determined quantitatively for coordinations almost free of any type of topologically induced distortion. This is clearly not the case for the Mn(3) and Mn(4) sites in braunite, whereas the equivalent O2 and O1 atoms within the square bases and on the apices of the Mn(2)O6 octahedra, respectively, allow the changes in the JT effect to be studied. The differences in the Mn-O1 and Mn-O2 bond compression indicate that the JT distortion slightly increases with pressure, which is in contrast to, and therefore competing with, the compressional changes in the topologically induced distortion.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page