

The validity of the Adam-Gibbs theory which relates viscosity to configurational entropy is now well established for the case of silicate melts. Using viscosity and heat capacity data obtained in this institute the Adam-Gibbs theory has been used to estimate configurational entropies quenched in at the glass transition (Sc(Tg)) for a number of simple silicate compositions. The compositional dependence of these variations is interpreted within the framework of current models of silicate melt structure, with the ultimate aim of providing a predictive model of silicate melt viscosity.
Viscosities of melts close to the join SiO2-NaAlSiO4 have been measured in the range 1-1012 Pa s using a combination of concentric cylinder and micropenetration techniques, including the first measurements of nepheline melt at temperatures close to its glass transition (Tg). The compositions range in SiO2 content from 50 to 82 mol%, and particular attention was paid to quantify the effect of small differences in Na/Al. Entropies of glasses along the join SiO2-NaAlSiO4 were estimated using heat capacity data available in the literature. Modelling of these entropies suggests that they may be divided into contributions from: i) mixing of Al and Si tetrahedral sites within an oxygen lattice (chemical contribution), and ii) variations in the topology of the oxygen network (topological contribution). The topological contribution is inferred to dominate, not only at the glass transition, but increasingly so at higher temperatures. It is further proposed that the calorimetric glass transition (at constant cooling rate) may occur when the topological contribution reaches a limiting value. The variation of the chemical contribution as a function of composition implies that Al-Si ordering increases as silica content decreases from SiO2 to nepheline. This conclusion is supported independently by phase equilibria, spectroscopic and calorimetric measurements.
The Adam-Gibbs theory was also applied to the case of additions of various alkali and alkaline earth elements to a haplogranitic base system. The fragility (defined as the gradient of the viscosity curve at Tg on a reduced temperature scale Tg/T) as a function of the Adam-Gibbs parameters is described by the following equation:
where m is the fragility, Ae is a constant and
DCp(Tg)
is the jump in heat capacity at the glass transition. Using the above equation,
and assuming a constant value of Ae for all melts,
heat capacity and viscosity data which we have obtained have been used
to estimate Sc(Tg).
The addition of alkali and alkaline earth elements results always in an
entropy increase as may be expected (Fig. 3.6-4). It is found that all
of the alkaline earth cations (with the exception of Ba) define a single
trend as equimolar additions are made. In the case of the alkali cations
each element defines its own trend, with Cs resulting in the greatest,
and Li the smallest increase in entropy per mole of cation added. The Li
data lie closest to the trend defined by the alkaline earth cations. If,
as is suggested by the study of the join SiO2-NaAlSiO4,
these entropies can be divided into a constant contribution from topological
entropy, plus a contribution related to the diversity of tetrahedral sites,
one may attempt to qualitatively explain the observed trends. The addition
of cations which cannot replace Na or K associated with Al in the haplogranitic
base composition gives rise to one new type of tetrahedral site, containing
a non-bridging oxygen (NBO) attached to the element being added. Equimolar
additions of different cations may therefore be expected to result in very
similar entropies, as is the case for the divalent cations (with the exception
of Ba). This observation therefore implies that these cations do not compete
effectively with Na or K for charge balancing roles, as may be expected.
If, however, an element is added which can replace Na and K, then a much
greater diversity of tetrahedral sites is produced, consisting of aluminate
tetrahedra charge-balanced by Na, K, and the element added. Additionally,
tetrahedra attached to NBO's of the added element, as well as Na and K
displaced from charge balancing roles are formed. The increase in entropy
due to the addition of a given cation may therefore be expected to be proportional
to the effectiveness of its competition for the charge-balancing roles.
The observed trends are therefore consistent with Cs being the most effective,
and Li the least effective of the alkali cations, as expected.
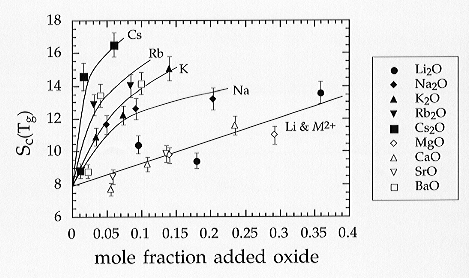 |
Fig 3.6-4: Estimated configurational entropies (J/mol-K) of glasses of haplogranitic composition (HPG8) with additions of various alkali and alkaline earth oxides, as shown |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page