

Silicate-carbonate liquid immiscibility has long been proposed as a possible mechanism for the origin of carbonatites associated with ultramafic alkaline rocks in complex intrusive bodies. Many field observations and data on fluid inclusions in minerals support the idea of liquid immiscibility in connection with carbonatite genesis. Modern experimental studies of silicate-carbonate liquid immiscibility started in the early sixties and since that time the separation of carbonatitic liquids by immiscibility from a carbonated silicate melt has been demonstrated for many synthetic systems and natural rock compositions. At the present time there seems to be no doubt that liquid immiscibility does occur in certain alkali-rich silicate-carbonate systems at crustal pressures, but more precise data on compositions of the conjugate melts and partitioning of major and trace elements is necessary for establishing a solid experimental basis for the immiscibility hypothesis.
Experimental studies in silicate-carbonate systems are hampered by high crystallization rates of carbonate-rich melts. Carbonatitic liquids usually do not quench to form glasses and this complicates chemical analyses and interpretation of run products. Study of trace element partitioning between the immiscible liquids is an even greater problem. It is not possible to avoid contamination by hand-picking separation of the immiscible globules in the run products, possibly explaining why the available data on trace element partitioning are limited and different analytical methods are not in good agreement.
The centrifuge separation of the immiscible liquids eliminates many
quenching problems and enables the avoidance of contamination when the
immiscible liquids are subjected to bulk chemical analyses. Thus, we have
used the rotating autoclave for centrifuge separation of immiscible silicate
and carbonate liquids. Two batch compositions have been studied. The first
is a five-component SiO2-Al2O3-CaO-Na2O-CO2
silicate-carbonate synthetic mixture. The second reactant mixture is similar
to the first, but is complicated by additional components: MgO, K2O
and P2O5. The starting mixtures were prepared from
aluminosilicate glass corresponding in composition to the nepheline - sodium
disilicate eutectic in the SiO2-Al2O3-Na2O
system and reagent-grade carbonates. The compositions of starting reactant
mixtures and immiscible liquids are listed in Table 3.6-1. The separation
has been performed at an acceleration of 500 g, and the duration of the
runs was 1 hour. The runs resulted in two distinct immiscible layers separated
by a sharp meniscus (Fig. 3.6-5).
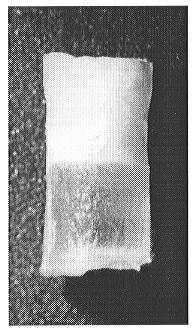 |
Fig 3.6-5: Carbonate and silicate liquids separated in a centrifuge autoclave. The sample is about 3 mm high. |
The lighter carbonate liquid layer, crystallized on quenching into a fine-grained aggregate, lies above the glassy silicate layer. The first batch composition produced also less than 1 wt% of tiny (about 5 microns) combeite quench crystals distributed throughout the silicate glass. The bulk chemical compositions of the layers have been analysed by ICP-AES and Cameca electron microprobe. Both methods are in good agreement.
|
|
|
||||||||||
|
|
|
|
|||||||||
| Batch |
|
|
|
Batch |
|
|
|
|
|
|
|
| SiO2 | 29.67 | 57.41 | 1.9 | 0.033 | 29.67 | 51.63 | 4.92 | 0.085 | 46.27 | 7.12 | 0.140 |
| Al2O3 | 5.02 | 9.00 | n.a. | - | 5.02 | 8.10 | n.a. | - | 7.31 | 0.26 | 0.033 |
| MgO | - | - | - | - | 2.67 | 3.39 | 1.36 | 0.358 | 3.47 | 1.26 | 0.330 |
| CaO | 11.21 | 8.03 | 14.29 | 1.779 | 6.96 | 3.97 | 10.14 | 2.274 | 5.66 | 9.02 | 1.448 |
| Na2O | 32.85 | 22.93 | 42.51 | 1.854 | 22.61 | 15.62 | 30.13 | 1.718 | 18.12 | 29.04 | 1.455 |
| K2O | - | - | - | - | 13.63 | 10.98 | 14.14 | 1.147 | 11.01 | 15.16 | 1.251 |
| P2O5 | - | - | - | - | 1.14 | 0.39 | 1.87 | 4.265 | 0.54 | 1.96 | 3.316 |
| CO2* | 21.25 | 2.64 | 41.30 | - | 18.29 | 5.92 | 37.44 | - | 7.62 | 36.16 | - |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page