

Recent high-pressure experiments and Mössbauer spectroscopy measurements suggest that silicate perovskite is the main reservoir for ferric iron and aluminum in peridotite composition lower mantle. In addition the coupled substitution of Al3+ and Fe3+ in silicate perovskite may have large effects on its crystal chemistry and physical properties. On the other hand, the deep subduction of Al-rich basaltic rock (e.g. mid-oceanic ridge basalt, MORB), which is an important component of subducting slabs, may result in the formation of additional Al-rich phases, particularly in the Earth's transition zone and lower mantle. The identification of such minerals is important because subducted oceanic crust may remain as a major long term chemical heterogeneity in the predominately peridotite mantle.
Multianvil press and laser heated diamond anvil cell experiments were carried out on natural MORB and aluminous garnet compositions under lower mantle conditions. In these experimental run products two Al-rich phases have been identified. The first isan orthorhombic Ca-ferrite structured phase with space group Pbnm, which has a composition on the MgAl2O4-NaAlSiO4 join. The second is hexagonal with space group P63/m and a stoichiometry also close to AB2O4. The end-member of this phase may have Ca and K on the A position and Al and Si on B, but in experiments on MORB composition its chemistry was more complex and also contained significant Mg and Fe. This phase, which we call NAL (new aluminous phase), also possesses a strong similarity to the Ca-ferrite structure and has also been observed coexisting with aluminous garnets. These Al-rich phases may have a high potential to accommodate ferric iron and alkalis, which appear to be essential cations for the stabilization of these phases. They may, therefore, influence the oxidation state and the alkali metal contents in heterogeneous regions of the lower mantle. However, the phase relations between the Al-rich phases and their crystal chemistry, including the iron valency, remains unclear.
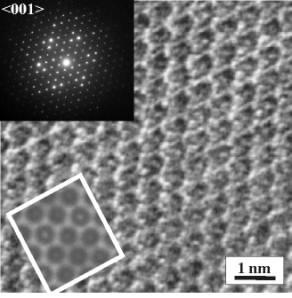
We have examined the Ca-ferrite phase and the hexagonal Al-rich phase (NAL) using a transmission electron microscope equipped with energy dispersive X-ray (EDX) and electron energy loss spectroscopy (EELS) systems. Although the powder X-ray diffraction pattern of the NAL phase is similar to the Ca-ferrite structure, the selected area diffraction patterns in the [001] beam direction reconfirmed the distinct difference between both structures. High resolution TEM of the NAL phase also shows the difference from the Ca-ferrite structure and the similarity to previous X-ray refined structures of the hexagonal phases (Fig. 3.3-13). From the results of EDX analyses and a comparison of the sizes of atoms on the various sites, the stoichiometry of the Al-rich phases is consistent with almost all iron being present as Fe3+. At present we are directly investigating the iron valency of these samples using the high resolution EELS technique. In the future we also hope to refine the phase relations in these aluminous systems to identify plausible phases that may reside in chemically heterogeneous regions of the Earth's mantle.
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page