

Diamonds are formed in the Earth at high pressure and temperature at conditions of relatively low oxygen fugacity. However, during their residence in the mantle and transport to the surface they can be exposed to oxidising conditions outside their stability field, leading to their corrosion and eventually to resorption. But where does such resorption occur? While some studies conclude that the majority of diamond resorption takes place in the oxidising environment of the kimberlite magma, other studies suggest that some resorption occurs already in the mantle lithosphere prior to entrainment. Such questions are of obvious economic importance since they can influence the evaluation of diamond deposits with regard to diamond grade.
Processes which can alter the chemistry of the mantle lithosphere include infiltration of silicate-rich liquids (melts) and/or hydrothermal fluids. Chemical changes occurring during the resulting metasomatism have been documented in studies of zoning in minerals using tools such as the electron, proton and ion microprobes, but due to the difficulty in determining the oxidation state of elements, particularly iron, only limited information is available on changes in oxygen fugacity during metasomatism.
Mössbauer spectroscopy is an excellent method to determine the oxidation state of iron, but the
conventional approach requires absorbers of approximately one cm in diameter.
A method developed at Bayerisches Geoinstitut, however, has improved spatial
resolution by two orders of magnitude, enabling absorbers as small as 50
µm to be studied. We applied this method to the determination of
Fe3+/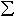 Fe values in zoned garnets from the Wesselton kimberlite,
where zoning has resulted from a sequence of metasomatic processes. Beam
sizes of 400 µm allowed an investigation of the garnet Fe3+/
Fe values in zoned garnets from the Wesselton kimberlite,
where zoning has resulted from a sequence of metasomatic processes. Beam
sizes of 400 µm allowed an investigation of the garnet Fe3+/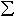 Fe
with respect to their cores, rims and secondary rims (Fig. 3.3-18a). Results
were then used in conjunction with co-existing olivine and orthopyroxene
compositions to determine oxygen fugacities using the olivine-orthopyroxene-garnet
oxybarometer.
Fe
with respect to their cores, rims and secondary rims (Fig. 3.3-18a). Results
were then used in conjunction with co-existing olivine and orthopyroxene
compositions to determine oxygen fugacities using the olivine-orthopyroxene-garnet
oxybarometer.
 |
Results show a progression in oxygen fugacity from relatively reduced conditions prior to metasomatism (determined from literature data of an unaltered sample from the same locality) to successively more oxidising conditions as metasomatism progresses (Fig. 3.3-18b). The increase in relative oxygen fugacity during metasomatism has some relevance to diamond preservation. The upper oxygen fugacity limit of graphite or diamond in harzburgites is defined by the reaction of olivine with diamond (or graphite) and oxygen to produce orthopyroxene and carbonate. Together with the graphite-diamond univariant equilibrium, this defines the maximum stability region of diamond as a function of pressure, temperature and oxygen fugacity in the peridotite system. The activity of carbonate in the system increases with increasing oxygen fugacity until the reaction boundary is reached, at which point the carbonate activity becomes unity. For diamond-bearing assemblages that undergo metasomatism, the degree of diamond preservation is likely influenced by the activity of carbonate in the metasomatic fluid. Increasing carbonate activity would increase the degree of diamond reaction with the fluid, hence causing resorption of the diamond even at oxygen fugacities below the reaction boundary. Fig. 3.3-18b illustrates two iso-activity curves for carbonate. While carbonate activities of 0.001 and 0.01 would not be expected to cause reaction of diamond with the fluid, higher activities could be expected to produce an observable reaction.
Many garnet peridotites from South Africa show relatively low oxygen fugacities, and many lie within the diamond stability field (open circles and squares in Fig. 3.3-18b). Diamond would hence be preserved during the initial stages of such metasomatism, up to an increase in relative oxygen fugacity of 1-2 log-bar units. Beyond that point, however, the fluid would react with the diamonds, leading to their oxidation and resorption.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page