

Although melting of the Earth's present day mantle is believed to occur principally at relatively shallow depths (<100 km), there were most likely periods in the Earth's early history when the mantle was molten down to lower mantle conditions. In addition mantle plumes in the Archaean mantle may also have commenced melting in the transition zone or lower mantle. In order to understand element fractionation during such lower mantle melting events we have performed experiments between 21 and 24 GPa to identify the liquidus to solidus phase relations and element partition coefficients for two peridotite bulk compositions. The starting materials, pyrolite doped with trace elements and an undoped KLB-1 peridotite, were prepared as oxide mixes, and contained in Re capsules. Multianvil experiments were carried out using 10/4 (octahedral edge length / anvil truncation edge length) and 18/8 pressure cell configurations with LaCrO3 heaters and axially inserted WRe thermocouples. The 18/8 configuration uses a stepped heater, and was run in the 5000 tonne multianvil press. This assembly was specifically developed in order to reduce the thermal gradients in these experiments and can reach pressures of 23 GPa at an applied load of 2000 tonnes. In some of the experiments Re-rod sample containers (Fig. 3.3-19) were prepared by spark erosion with two parallel sample holes, 0.3 mm wide and 0.4 mm deep, allowing two different compositions to be run at identical conditions.
 |
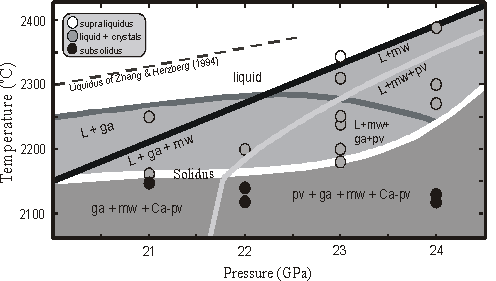
The melting relations of the two compositions (pyrolite and KLB-1) are almost identical in the 21-24 GPa range (Fig. 3.3-20). Garnet (ga) is on the liquidus between 21-22 GPa and changes to magnesiowüstite above 23 GPa. The crystallization sequence changes from ga, mw, Ca-perovskite (Ca-pv) at 21 GPa to mw, pv, ga, Ca-pv at 24 GPa. The solidus and liquidus temperatures estimated for this pressure range are 50-100 K lower than the values derived for the KLB-1 composition by Zhang and Herzberg (JGR 99, 17729, 1994). FTIR spectroscopy on a thin section containing melt and a subsolidus crystalline assemblage did not show evidence for the presence of OH- in either the solid or liquid regions. This suggests that H2O was probably not present in concentrations high enough to significantly affect the melting temperatures in these experiments.
Major element mineral-melt partitioning shows only minor variations with pressure in the investigated range. The mineral-melt KDFe/Mg for magnesiowüstite decreases with pressure slightly while values for garnet and perovskite increase. The Ca/Al-ratio is always elevated in the melt phase. Cr changes from being slightly incompatible to compatible in both garnet and magnesiowüstite between 21 and 22 GPa, whereas Cr is slightly incompatible in perovskite. Ni remains highly compatible in magnesiowüstite and incompatible in garnet and perovskite in the entire pressure range. The partitioning coefficient for Ti increases systematically with pressure for all of the phases, and Ti is compatible in perovskite.
 |
Mössbauer spectroscopy of a run product from 23 GPa, which contained approximately 50% quenched
melt, revealed 28% Fe3+/ Fe in the crystals
and 15% Fe3+/
Fe in the crystals
and 15% Fe3+/ Fe
in the melt, the tentative conclusion being that Fe3+ may be
slightly compatible in the solid assemblage during melting at these conditions.
Perovskite is probably the main reservoir of Fe3+ in these run
products. If all Fe is assumed to be ferrous, an average perovskite formula
normalized to 12 oxygens has a cation sum of 8.07. This cation excess can
be accounted for by the presence of about 85% ferric iron in the perovskites.
Alternatively, the apparent excess of cations may be a result of an oxygen
deficient perovskite component (up to about 10% of the Mg4Al2Si2O11-component).
EELS analyses of the Fe3+ distribution between the solid phases
in these experiments are underway.
Fe
in the melt, the tentative conclusion being that Fe3+ may be
slightly compatible in the solid assemblage during melting at these conditions.
Perovskite is probably the main reservoir of Fe3+ in these run
products. If all Fe is assumed to be ferrous, an average perovskite formula
normalized to 12 oxygens has a cation sum of 8.07. This cation excess can
be accounted for by the presence of about 85% ferric iron in the perovskites.
Alternatively, the apparent excess of cations may be a result of an oxygen
deficient perovskite component (up to about 10% of the Mg4Al2Si2O11-component).
EELS analyses of the Fe3+ distribution between the solid phases
in these experiments are underway.
Below the solidus at 21 GPa ringwoodite was not observed, but instead an assemblage of majorite, Ca-perovskite and magnesiowüstite was identified. The disappearance of ringwoodite in the peridotite assemblage at elevated temperatures due to the reaction ringwoodite = majorite garnet + mw may have implications for the dynamics of mantle up-welling. In the past it has been suggested that the negative Clapeyron slope of the pv + mw = ringwoodite reaction may inhibit whole mantle convection. Our results imply, however, that at higher temperatures upwelling plumes may not encounter this reaction, but may instead go through a transformation from perovskite to majorite, which has been shown experimentally to have a positive Clapeyron slope.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page