

Wadsleyite (ß-(Mg,Fe)2SiO4)
is probably the most abundant mineral in the upper part of the transition
zone, between depths of 410 and 520 km. The seismic discontinuity which
is observed at 410 km is thought to result from the transformation of olivine
(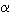 -(Mg,Fe)2SiO4) to wadsleyite,
and the exact depth and width of this transition are related to the physical
and chemical properties of the two phases. Two important factors that could
control the width of this discontinuity are the concentrations of dissolved
water in the two phases and the degree of ordering of cations, vacancies
and hydroxyl defects. In this study we have obtained 1H MAS
NMR and FTIR spectroscopic information on the ordering of OH in wadsleyite
and we show how, at room temperature, the ordering is strongly dependent
on the total dissolved water concentration.
-(Mg,Fe)2SiO4) to wadsleyite,
and the exact depth and width of this transition are related to the physical
and chemical properties of the two phases. Two important factors that could
control the width of this discontinuity are the concentrations of dissolved
water in the two phases and the degree of ordering of cations, vacancies
and hydroxyl defects. In this study we have obtained 1H MAS
NMR and FTIR spectroscopic information on the ordering of OH in wadsleyite
and we show how, at room temperature, the ordering is strongly dependent
on the total dissolved water concentration.
The synthesis of hydrous wadsleyite was performed using the 5000 tonne multianvil apparatus with an 18 mm edge length octahedral pressure assembly and 11 mm truncations on the WC cubes (18/11 configuration). With such an assembly, the large volumes of hydrous wadsleyite could be produced that were necessary for collecting high quality 1H MAS NMR spectra. Starting powders were placed inside platinum capsules fabricated from 2 and 3 mm outside diameter, 0.1 mm wall thickness tubes. The total capsule length was 3.5 mm. The 18/11 configuration enables a pressure of 15 GPa to be reached with the application of approximately 900 tonnes. After pressurization the samples were heated for 3 hours and then quenched by switching off the power.
FTIR spectra were obtained using a Nicolet 800 FTIR at the University of Bristol with an unpolarized IR source. For some samples the crystals grown in the synthesis experiments were large enough to perform single crystal IR measurements. The infrared spectra of selected single crystals provide abundant information on the number and location of OH species in hydrous wadsleyite. In principle the orientation of OH dipoles can also be determined by orienting crystals, even for unpolarized light. However, in practice it was found that for samples with greater than about 1 wt% H2O, the OH absorption is so strong that the crystals have to be less than 30 µm thick, and polishing and manipulating crystals of this size is very difficult. 1H MAS NMR spectra were obtained using a Bruker MSL 300 spectrometer and either a custom-built probe based on a Doty 5mm supersonic spinning assembly or a Bruker 4 mm MAS probe.
Infrared spectra consist of a series of narrow bands between 3530 and 3800 cm-1, a group of peaks in the range 3200-3450 cm-1 and two broad low frequency features which clearly increase in intensity with increasing dissolved water concentration. The 1H MAS NMR spectrum consists of a broad band containing several partially resolved features that can be fitted with 6 peaks with chemical shifts between 1.5 and 11 ppm (Fig. 3.4-2).
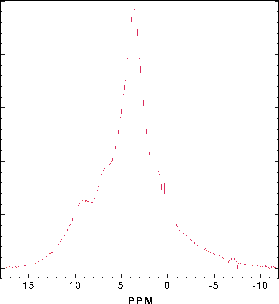 |
The peak positions in the FTIR and NMR spectra of hydrous wadsleyite were compared with the known correlations between O-H·O distances and O-H stretching frequency. In both cases quite good agreement was found, and in most cases the IR and NMR peaks could be confidently assigned. The surprising conclusion is that for samples of hydrous wadsleyite containing around percent level concentrations of dissolved H2O, the hydroxyl is highly disordered; O1 is not the only site for protonation. For samples with low water concentration O1 and O2 are the most likely sites for protonation. At the high temperatures of the transition zone it is likely that hydroxyl is more disordered than under ambient conditions, so the best thermodynamic model for hydrous wadsleyite may involve complete disorder of hydroxyl over all possible sites.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page