

Carbonates are important solutes in the mantle fluids that are responsible for metasomatic processes.
However, the constitution of such fluids at pressures and temperatures
above 2 kbar and 500oC is virtually unknown. Accordingly, the
first in-situ Raman spectra of Na, K, and Ca-carbonate solutions
were measured up to 15 kbar and 800oC. The experiments were
carried out using an externally heated diamond-anvil cell together with
a DILOR XY Raman spectrometer equipped with large CCD detector and an Ar+
laser (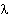 = 514.5 nm).
= 514.5 nm).
Initially, aqueous fluids in equilibrium with calcite in the temperature range 200-800oC and pressures from 1 to 15.4 kbar were studied. No Raman bands from carbonate ions in the solutions were observed. This is very surprising, because calcite is appreciably soluble under these conditions and because the carbonate ion is strongly Raman-active. This observation may therefore be related to some unusual speciation of dissolved CaCO3.
In order to study the properties of carbonate bearing aqueous fluids, we have measured Na- and K-bearing
carbonate solutions with different concentrations. At ambient pressure
and temperature using 90o scattering geometry, the v1(A1')
Raman band of CO32-was observed at about 1065 cm-1
even in dilute solution with a concentration of 0.0283 mol/kg. The other
two Raman active modes of CO3 2- at about 680 cm-1
v4(E') ) and at about 1410 cm-1
v3(E')) were not observed even in
solutions with 30 wt% Na2CO3 ( m = 2.83 mol/kg).
The depolarization ratio of the v1(A1')
band is very close to zero (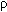 ~ 0.03 - 0.08).
Accordingly, at ambient conditions the broadening of the polarized band
is related mainly to vibrational relaxation. The broadening of the v1(A1')
band increases non-linearly with the Na-carbonate concentration. The vibrational
relaxation time
~ 0.03 - 0.08).
Accordingly, at ambient conditions the broadening of the polarized band
is related mainly to vibrational relaxation. The broadening of the v1(A1')
band increases non-linearly with the Na-carbonate concentration. The vibrational
relaxation time 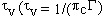 , where
c is the speed of light and
, where
c is the speed of light and
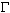 is the full width
at half maximum of the isotropic spectrum Iiso = IVV
- (4/3)IVH) is about 1.26±0.03
ps in a K2CO3 solution with a concentration of 0.940
mol/kg and 1.21±0.03 ps in a Na2CO3
solution with 0.940 mol/kg and increases with decreasing Na-carbonate concentration.
The
is the full width
at half maximum of the isotropic spectrum Iiso = IVV
- (4/3)IVH) is about 1.26±0.03
ps in a K2CO3 solution with a concentration of 0.940
mol/kg and 1.21±0.03 ps in a Na2CO3
solution with 0.940 mol/kg and increases with decreasing Na-carbonate concentration.
The 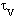 values for the K- and Na-carbonate
solutions studied are in very good agreement with published data.
values for the K- and Na-carbonate
solutions studied are in very good agreement with published data.
Since the scattering volume in the diamond-anvil cell is much smaller than for measurements in 90o geometry we have performed DAC measurements with Na- and K-bearing carbonate solutions of relatively high concentrations (m=0.28 and 0.94 mol/kg). Figure 3.4-10 shows
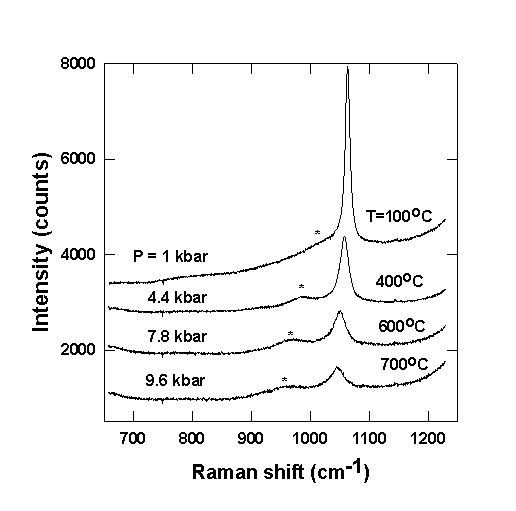 |
typical unpolarized raw Raman spectra of a Na-carbonate solution (m = 0.94 mol/kg) with a bulk water density of about 0.95 g/cm3 from 100 to 700 oC. This corresponds to pressures between 1 kbar at 100oC and 9.6 kbar at 700oC. At low temperatures and pressures only one strong peak at about 1065 cm-1 is observed, which is very close to the position of the v1(A1') band of 'free' CO3 2-. With increasing temperature and pressure three effects can be distinguished: (i) A new band at about 980 cm-1 starts to develop. Initially its intensity increases at the expense of the 1065 cm-1 band; (ii) the positions of the two bands shift to lower frequencies and their FWHM increases; (iii) at still higher temperatures and pressures (depending on the actual density of the solution) the intensity of both peaks starts to decrease. All three effects are reversible on cooling down the DAC. The same qualitative behaviour of the Raman spectra is observed in K2CO3 solutions with m = 0.94 mol/kg as well as in Na-carbonate solutions with m = 0.283 mol/kg and thus is not strongly composition dependent.
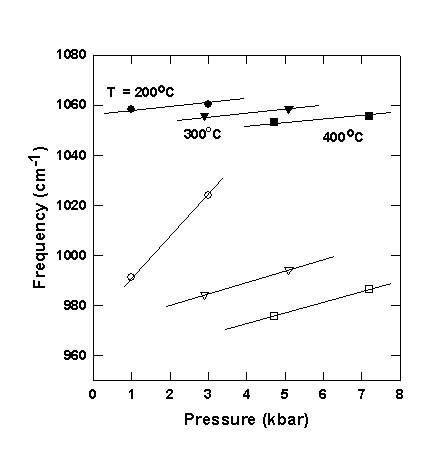
Combining data from experiments with different water densities we have separated the effects of pressure and temperature on the position of the two bands for Na-carbonate solution with m = 0.94 mol/kg (see Fig. 3.4-11). Similar effects are observed for both Na- and K-bearing carbonate solutions with different concentrations. The position of both bands increases with pressure at constant temperature and decreases with temperature at constant pressure, as would be expected for anharmonic vibrations. However, the anharmonicity and softening of the 980 cm-1 band is much stronger.
 |
We attribute the observed changes in the Raman spectra to the formation of MCO3- and/or neutral M2CO3 species in the solution. Ionic association has long been assumed in concentrated carbonate solutions even at ambient conditions. This would lead to a decrease of the molecular symmetry of the carbonate group from D3h to C2v or Cs. The symmetry lowering itself should lead to splitting of the doubly-degenerate E' modes into pairs of A1 - B2 modes. However, the E' modes are generally weakly Raman active and were not observed in the present study.
Normal mode calculations using valence type potential of monodentate MCO3- and M2CO3 molecules as well as bidentate M2CO3 molecules with C2v point group of symmetry have shown that M+ - O interactions alone lead only to small frequency shifts of the v1(A1') band while significant frequency shifts are to be expected if C-O bond weaking is introduced.Therefore, we attribute the 980 cm-1 band to the v1(A1) mode of CO3 species with significantly weaker C-O bonds due to M+ - CO3 2- association while the band at about 1060-1065 cm-1 to almost free CO3 groups. Previous ab-initio MO calculations at the HF 6-31G* level of Na2CO'O2 and NaCO'O2- molecules do predicted an increase of the unique C-O' bond length of about 0.8 Å but the calculated shift of the v1(A1) mode compared to the v1(A1') mode of free CO3 was rather small.
The behaviour of the intensity ratio R of the 980 to the 1065 cm-1 bands for Na- and K-carbonate solutions with concentration m = 0.94 mol/kg suggests that both temperature and pressure affect the speciation. At relatively low temperatures (100-300 oC) R is practically constant as a function of pressure. At higher temperatures (T ~ 400 oC) R starts to increase with pressure. At constant pressure the ratio R increases strongly with temperature.
The simultaneous decrease
of the intensity of the two Raman bands above 400-500 oC and
4-8 kbar pressures may be due to the formation of a new species not yet
detected in the Raman spectra. Alternatively, it might be explained by
(i) vibrational relaxation - dissipation of the vibrational energy of the
v1 mode into translational-rotational
motions of the surroundings. The increase of 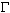 at constant pressure with temperature would be consistent with this interpretation;
(ii) decrease of the C-O bond polarizability, a corresponding decrease
of the C-O covalency and the number of non-bonding electrons due to the
ionic association.
at constant pressure with temperature would be consistent with this interpretation;
(ii) decrease of the C-O bond polarizability, a corresponding decrease
of the C-O covalency and the number of non-bonding electrons due to the
ionic association.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page