

Uni-directional chemical diffusion in a liquid with n components is described by the generalized Fick's laws
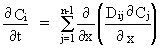
where the Dij are diffusion coefficients, Ci is the concentration of a component, t is time and x is a space coordinate. It is clear from this equation that it is impossible to predict quantitatively the nature and rate of diffusive fluxes in multicomponent liquids without a detailed knowledge of the matrix of diffusion coefficients represented by Dij. The melt compositions of geological interest in which the Dij are presently known are restricted to the systems K2O-Al2O3-SiO2 and CaO-MgO-Al2O3-SiO2. We have conducted interdiffusion experiments in the five-component system H2O-Na2O-K2O-Al2O3-SiO2 in an effort to determine the full matrix of diffusion coefficients for a hydrous melt near the granite minimum (HPG8: 79.1 % SiO2, 12.1 % Al2O3, 4.6 % Na2O, 4.2 % K2O) at 10 kbar pressure and at temperatures of 1300 °C and 1600 °C. Experiments were conducted by placing glass powders in 5mm Pt capsules with measured quantities of H2O; interfaces were tamped flat. Couples were composed in such a way that each couple intersected at the composition of HPG8 with 3 wt% additional water; experiments crossing this composition through five different vectors in the compositional space were conducted at each temperature to ensure that the experiments spanned all of the eigenvectors of the diffusion coefficient matrix.
A couple with an initial gradient in water concentration but negligible gradients in all other components showed no measurable diffusive flux of any component other that H2O itself. This indicates that the diffusion of H2O is relatively decoupled from that of the other four components in the system (pseudobinary), resulting solely in a counterflux of the other melt components by the Kirkendall effect. A reconnaissance experiment conducted in the analogous anhydrous system revealed that the forms of the diffusion profiles remain unchanged in dry melts, but the magnitude of the diffusion coefficients diminishes markedly in this much more viscous system, indicating a strong control on diffusivities by bulk water content. Diffusive fluxes of all other components in our system are strongly coupled, both within and between the two groups of elements present, i.e., network modifiers Na2O and K2O and network formers Al2O3 and SiO2. Uphill diffusion of all four of these components has been observed, with the effects on Na2O being particularly pronounced.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page