

Like other thermodynamic properties, the density of aluminosilicate melts is significantly affected by both composition and temperature. In an effort to provide as complete a description of melt density behaviour as possible, previous density determinations covering a very wide compositional range in the CaO-Al2O3-SiO2 system have been complemented by investigations in the MgO-Al2O3-SiO2 system and in the quaternary CaO-MgO-Al2O3-SiO2 system. For this purpose, compositions lying along the SiO2-Mg3Al2O6, SiO2-enstatite, enstatite-wollastonite and diopside-Al2O3 joins were selected.
Density measurements have been performed using both the Pt- and Ir-based double bob Archimedean methods. Densities have been determined for aluminosilicate liquids in the temperature range of the liquidus up to 1800 °C. The variations of the experimental densities with temperature of the investigated samples, range from 2.42 to 2.60 g/cm3.
The experimental results of this study, expressed in the form of the molar volume, are shown for compositions along the joins noted above in Fig. 3.7-5. Within the temperature and composition ranges investigated, the molar volume of the present melts in the MgO-Al2O3-SiO2 system (Fig. 3.7-5a), along the enstatite-wollastonite join (Fig. 3.7-5b), behaves linearly as a function of composition. However, an interaction between the alkaline-Earth oxide and SiO2 components, noted previously for Ca-aluminosilicate melts, was not observed in the case of Mg-aluminosilicate melts, possibly because the access to low SiO2-content liquids was not possible in the MgO-Al2O3-SiO2 system. The situation is more ambiguous for the diopside-Al2O3 join and depends on the density of pure liquid Al2O3 (see Fig 3.7-5c).
The partial molar volume and expansivity of each oxide component were
obtained by regression of the present experimentally determined molar volumes
at several temperatures including data from previous work. The parameters
returned from these fits show differences with published calculation schemes.
The partial molar volume at 1873 K disagrees by around 2 % for SiO2
and Al2O3, whereas the partial molar volume of CaO
and MgO from both models are very different (about 18 % and 6 %, respectively).
The partial molar volume of SiO2 obtained in this study compared
with the molar volume of pure SiO2 liquid exhibits a difference,
which becomes more pronounced at temperatures higher than 2200 K (> 2 %).
As the thermal expansion in these two studies is different, no disagreement
greater than 1 % is found for a temperature range from 1700 to 2100 K.
The partial molar volume of Al2O3 presents a negative
coefficient of thermal expansion, in disagreement with all the literature
data for liquid Al2O3.
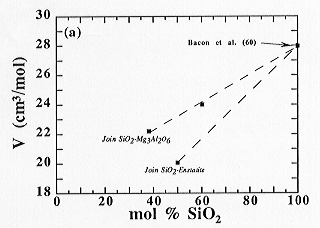 |
Fig. 3.7-5: Molar volume calculated at 1973 K: (a) along joins in the MgO-Al2O3-SiO2 system |
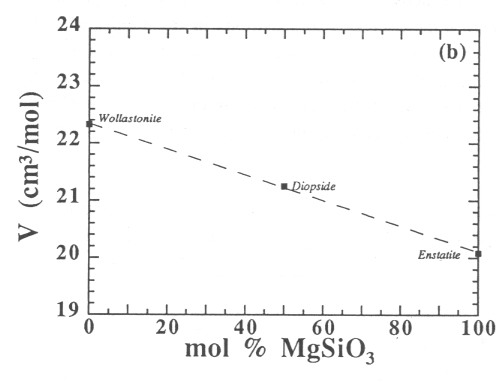 |
(b) along the wollastonite-enstatite join |
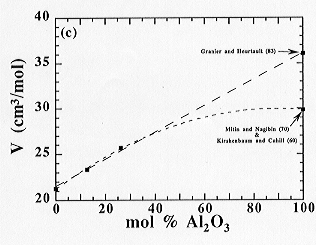 |
(c) along the diopside-Al2O3 join in the CaO-MgO-Al2O3-SiO2 system |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page