

The coordination sphere of transition metal ions like Ni2+ affects partition coefficients of these elements between melt and crystalline phases. The local environments of transition metal ions, and thus partition coefficients, are very sensitive to the degree of polymerization in the melt. As shown by earlier studies, dissolved water modifies aluminosilicate melt structure dramatically by reacting with bridging oxygens to form hydroxyl groups and thus depolymerizes the melt. We have investigated the influence of dissolved water on the coordination and crystal field splitting of transition metal ions in quenched albitic melts (glasses).
Dry albite glass containing 0.86 wt% NiO was prepared in a high temperature
furnace and bubble free hydrous NiO-doped glass was synthesized in the
presence of 5 wt% H2O in a Pt capsule in an externally heated
TZM bomb. The glass changed from a brownish colour to light green upon
hydration. UV/VIS microspectroscopy was used on doubly polished glass plates
to measure the crystal field spectra of the Ni doped glass chips. First
measurements show distinct differences of the spectra of the dry and the
hydrated Ni doped glasses (Fig. 3.8-8). The absorption band of the dry
Ni doped glass consists of three components: two
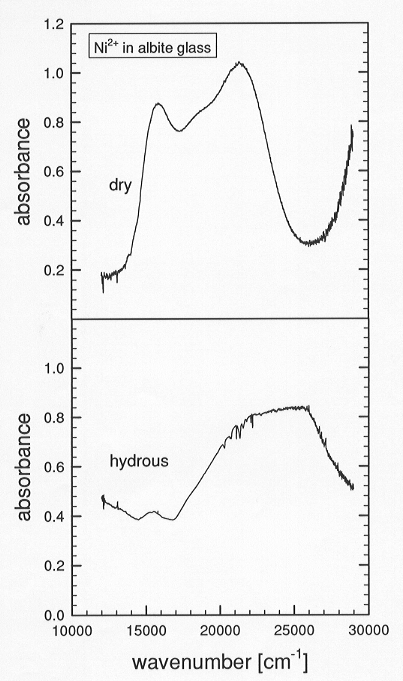 |
Fig. 3.8-8: UV/VIS spectra of dry and hydrous albite glasses. |
bands near 20,000 cm-1 are due to distorted octahedral Ni2+ and a third component near 15,600 cm-1 could be attributed to distorted octahedral and/or a small amount of tetrahedral Ni2+. In the spectrum of the hydrated glass, the intensity of the low energy component decreases dramatically and the peak due to octahedral Ni2+ is shifted to higher energies such that the band splitting due to the distortion of the octahedral sites is no longer perceptible. These results suggest that the dry albite glass consists of mostly Ni2+ in strongly distorted octahedral coordination and a small amount in tetrahedral coordination, whereas in the hydrated glass, Ni2+ occupies regular octahedral sites and a small amount of tetrahedral sites. The crystal field splitting energy is higher for the less polymerized hydrous glass, suggesting that increasing depolymerization of the melt by hydration leads to enrichment of transition metal ions into the melt phase.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page