

The "siderophile element anomaly" involves the highly siderophile elements (HSE) Ir, Pt, Rh and Re as well as the moderately siderophile elements Ni, Co, W and Mo. We have addressed this problem first at atmospheric pressure where reliable partition data can be obtained as a of oxygen fugacity.
For this purpose the newly designed "mechanically assisted equilibration method" was applied successfully during the last three years. It consists of a crucible filled with a haplobasaltic melt of An-Di eutectic composition which is equilibrated with the metal of the crucible. To accelerate the attainment of equilibrium the melt is constantly stirred with a spindle, machined from the same high purity metal as the crucible.
Stirring also reduces the problem of microparticle formation under reducing conditions. During the last year experiments were performed at extreme oxygen fugacities, controlled by C-containing and C-free gas mixtures, to extend the data set and to clarify the "carbonyl problem" (Annual Report, 1995).
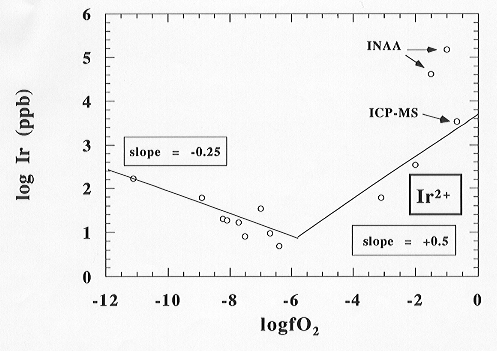
For the highly siderophile element Ir, experiments were carried out at very high oxygen fugacities using O2-Ar gas mixtures. Because of the long period required to wait following irradiation (˜ 2 to 4 months), the final results from Instrumental Neutron Activation Analysis (INAA) analyses performed in Mainz were obtained shortly before the present report and are shown in Fig. 3.2-2. This figure shows the typical v-shaped solubility behaviour for HSE at the investigated oxygen fugacities, as explained below. The new results confirm the previously observed trend (Annual Report, 1995). At very high oxygen fugacities solubility increases with increasing oxygen fugacity above 10-6. INAA results appear to deviate from a regression that would correspond to Ir2+ (slope of 0.5) above an oxygen fugacity of 10-2.
 |
|
|
Parallel analyses of the same samples performed by ICP-MS techniques in Canberra (Australia) fits perfectly with the 0.5 regression line proposed for Ir2+ in the melt. The ICP-MS analyses deviate from the INAA results by two orders of magnitude; e.g. a sample determined by neutron activation analysis showing an Ir content of 456,000 ppb gave 3479 ppb determined by ICP-MS techniques. This does not reflect the error in a single analysis, but is a difference between two analytically different techniques applied to identical samples. The difference might be explained as follows: INAA analyses were made on about 100 mg of a material chosen only optically (by eye) and the bulk concentration of Ir was determined, including all contaminations inside this sample material. In contrast, laser ablation ICP-MS is able to measure sample material near the surface, avoiding contaminated regions which tend to occur deep inside the sample.
Accepting this explanation the final results show at fairly high oxygen fugacities (above log fO2 = -6) an increase of the solubility with increasing fO2 as expected from theory. The equilibrium solubility data (in ppb) plotted versus the oxygen fugacity applied in the experiment (in double logarithmic units) can be correlated by a regression line with a slope of +0.5. This slope indicates a 2+ formal valence state of Ir in the melt structure at the investigated P, T and fO2 conditions (1 atm; 1340°C; -5 < log fO2 < ±0). The range of the solubilities lies between 50,000 ppb at high oxygen fugacity and only 3 ppb around log fO2 = -5, which is the minimum solubility reported to date.
A further decrease of the fO2 (-12 < log fO2 < -5) applying CO/CO2 gas mixtures leads to a sudden increase in the equilibrium solubilities of Ir in the melt from 3 ppb up to about 180 ppb at an oxygen fugacity of 10-11. This observation might be due to a possible C-interaction or formation of carbonyls of Ir in the melt structure, increasing the solubility of Ir in the melt during this process. Elucidation of the processes occurring in the melt is presently not possible because concentrations are too low for spectroscopic investigations.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page