

The rare earth elements (REE, from 57La to 71Lu) have been found to be a powerful tool for the evaluation of processes in ultramafic rocks. The partition coefficients of REE between phases of the upper mantle, especially between clinopyroxene and garnet, are temperature dependent. A compositional dependence in rocks with different contents of CaO and Al2O3 can also be expected.
The project focuses on the determination of the dependence of empirical partition coefficients of REE between upper mantle phases on temperature, pressure, and composition in absence of melts. To get maximum information on the partition behaviour the elements La, Nd, Sm, Gd, Ho, Yb were selected, because in clinopyroxenes light REE (La to Eu) are preferably substituted, whereas in garnets the heavy REE (Gd to Lu) enter the structure more easily.
The initial experiments were performed on the CaO-free system MgO-Al2O3-SiO2-REE2O3 to determine phase stabilities and the maximum REE concentrations in olivine, orthopyroxene, garnet and spinel. This was a prerequisite to optimize conditions for electron probe microanalysis. To avoid line overlaps in the generated X-ray emission spectra the experiments were doped with only one REE each.
The experiments were run at 1400°C, 2.5 GPa and 1200°C, 3.0
GPa. The phases which formed with the highest REE concentrations were a
REE-silicate (oxy-apatite) or a garnet solid solution, depending on the
stability of the specific REE garnet phase. The REE-silicate phase may
contain up to 10 wt% Al2O3 and 5 wt% MgO, and in
the Al2O3-free system up to 9 wt% MgO. This phase
subtracted SiO2 from the bulk composition such that olivine
or spinel were formed instead of the expected orthopyroxene. The maximum
contents of REE substituted into olivine and spinel range between 0.1 and
0.5 wt% REE2O3 and show a systematic increase with
increasing atomic number of the REE. For orthopyroxene the contents for
Ho2O3, Yb2O3 and Lu2O3
are ca. 1.5 wt% at 1400°C, but ca. 1 wt% at 1200°C. The garnet
compositions in the Al2O3-bearing system are all
solid solutions, whereas the garnet in the Al2O3-free
system seems to be a pure phase. Therefore the differences between the
partition coefficients for REE between garnet and orthopyroxene can have
contributions from both temperature and Al2O3 content.
In one experiment doped with Sm the partition coefficient could be calculated
for orthopyroxene coexisting with pyrope (see Fig 3.2-4).
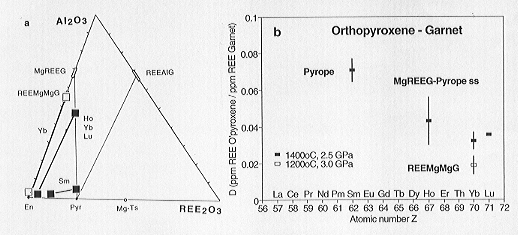 |
Fig. 3.2-4: a) Compositions of synthesized orthopyroxenes and garnets in the section Enstatite - Al2O3 - REE2O3 of the system MgO - Al2O3 - REE2O3 - SiO2 (wt%). Abbreviations: En: Enstatite, Pyr: Pyrope, Mg-Ts: MgAlAlSiO6, REEAlG: REE3Al5O12, MgREEG: Mg2.5REE3Si2.5O12, REEMgMgG: (REE2Mg)Mg2Si3O12. |
As a result of the experiments, it is concluded that multiple buffering for SiO2 and Al2O3 in the presence of REE-silicate is needed. Therefore a garnet lherzolite composition (20% olivine, 32% orthopyroxene, 24% clinopyroxene, 24% garnet, all in wt%) was chosen for the current experiments. In the system CaO - MgO - Al2O3 - SiO2, SiO2 will be buffered by olivine - orthopyroxene, and Al2O3 by garnet - orthopyroxene. Two isotherms at 1200°C and 1400°C, 3 GPa are being determined at 10 wt% REE2O3 (added as REE-silicate) and will later be determined at lower REE concentration. Additionally compositional effects of Al2O3 and CaO are being investigated.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page