

Although not normally found as structural elements in crystalline phases, silicon coordinated by five oxygen atoms plays a central role in a large number of dynamic processes that occur in silicates. It is probably a component in aluminosilicate glasses and melts at the pressures and temperatures of the Earth's mantle in which it dominates their transport properties. It is believed to exist in the materials produced by non-hydrostatic compression of silicate minerals such as quartz, in the glasses produced during pressure quenching of high-pressure silicates such as CaSiO3 perovskite, and as an intermediate activated state during oxygen diffusion in silicate minerals. This year we have synthesised a high-pressure phase of CaSi2O5 and have undertaken a full structure determination by single-crystal diffraction which demonstrates that this is the first inorganic crystalline phase to contain SiO5 groups as an essential component of the structure.
We synthesised CaSi2O5 from a glass of the same composition loaded into a rhenium capsule and held at 11 GPa and 1350°C for 4 hours in the 1,000 ton multianvil press. X-ray intensity data were obtained from several single crystals selected from the run products, most of which were twinned. This twinning arises from a symmetry change from monoclinic to triclinic that occurs on pressure release following synthesis. From one untwinned crystal we were able to obtain intensity data and solve the structure by direct methods
The refined structure of CaSi2O5 has the same
topology as that of titanite CaTiSiO5 from which it can be derived
by substitution of the smaller Si cation for the Ti in octahedral coordination,
together with mostly small displacements of the atoms and a small distortion
of the lattice from monoclinic symmetry. Thus in CaSi2O5
the calcium remains in a cage site which is coordinated by 8 oxygen atoms,
and there are chains of Si-O polyhedra which run parallel to the a-axis
which are internally bridged by SiO4 tetrahedra (Fig. 3.3-1).
However, these chains contain three symmetrically distinct silicon sites
in place of the single distinct TiO6 octahedron in titanite
itself. Two of the silicon sites in CaSi2O5 are also
octahedrally coordinated, with bond lengths and angles typical of SiO6
octahedra in other high-pressure silicate structures. However, the third
silicon is substantially displaced from its "ideal" position, and one of
the coordinating oxygen atoms is in addition moved to a distance of 2.8305(31)
Å from the silicon. The calculated bond valence for this Si-O distance
is 0.04 indicating that there is no significant bonding between the silicon
and this oxygen. This silicon site is therefore coordinated by five oxygens,
and CaSi2O5 is the first inorganic crystalline phase
proved by structural analysis to contain SiO5 groups as an integral
part of its structure.
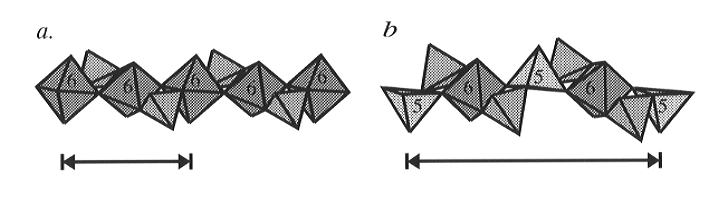 |
Fig. 3.3-1: Polyhedral representation of the polyhedral chains which run parallel to the crystallographic a-axis in (a) high-temperature titanite and (b) CaSi2O5. The coordination of the Ti atoms in (a) and the atoms in (b) are indicated by the roman numerals. The alternation of 5- and 6-coordinated Si in the structure of CaSi2O5 results in a doubling of the length of the a-cell parameter relative to that of titanite, as indicated. All of the tetrahedra shown are occupied by silicon. |
Inspection of Fig. 3.3-2 clearly shows the structural relaxation that
has occurred upon "removal" of the sixth oxygen from the coordination sphere
of the silicon. The oxygen opposite has moved in towards the silicon, and
the basal oxygens have rotated towards the vacated position of the sixth
oxygen. This relationship between the geometry of the SiO5 group
and that of the octahedral groups of SiO6 in CaSi2O5
immediately suggests that similar coordination polyhedra for pentacoordinate
silicon might be expected to form upon the creation of oxygen vacancies
in high-pressure silicates whose normal structure contains SiO6
octahedra. Such defects are
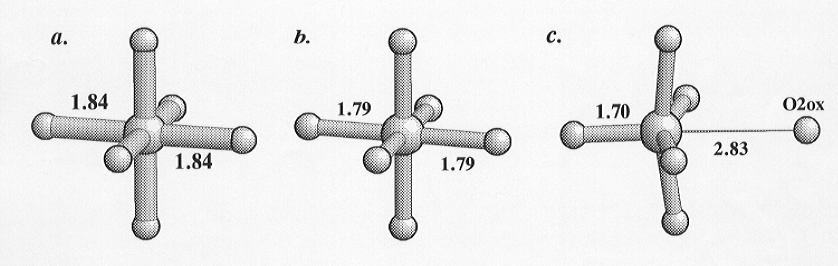 |
Fig. 3.3-2: The coordination of the three Si sites in CaSi2O5. Comparison of (c) with the Si sites with 6-coordination in (a) and (b) indicates the distortion arising from the reduction of the coordination to 5-fold as a result of the removal of the O2ox oxygen. Numbers are interatomic distances in Ångstroms. |
expected to play a central role in oxygen diffusion in the dominant phase of the Earth's lower mantle, (Mg,Fe)SiO3 perovskite. The detailed geometry that we have determined for such a SiO5 group will allow more realistic calculations to be performed on the energetics of the formation of such defects and their role in determining diffusion rates in the Earth's lower mantle, as well as their possible role in amorphisation of high-pressure silicates (such as CaSiO3 perovskite) induced by pressure release. The presence of pentacoordinate silicon in glasses and melts has previously been inferred from the observation of signals at approximately -150ppm in 29Si MAS-NMR spectra, and the observation of a similar signal from CaSi2O5 coupled with our structural analysis confirms this assignment. This study therefore also opens up the possibility of the quantification of pentacoordinate silicon in glasses and its possible influence on network ion diffusivities and associated viscosity reduction in silicate melts at high pressures.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page