

Carbonatitic melts are the most volatile-rich terrestrial magmas. Since alkali and alkaline earth carbonates are the main components of carbonatite magmas, the concentration of carbon dioxide in these melts is estimated to be about 30-40 wt%. Water is also highly soluble in carbonatite melts and may be present in large amounts. Observations of carbonatite eruptions, bulk rock chemistry, studies of fluid inclusions trapped in minerals, the abundance of hydrous silicates in carbonatites as well as metasomatic alteration of country rocks suggest that water and aqueous fluids play a major role in carbonatite magmatism.
Separation of supercritical aqueous fluids in shallow-level magma chambers has long been proposed to be one of the main processes responsible for the chemical diversity of carbonatites. These fluids may extract large amounts of highly soluble alkali carbonates and change the bulk composition of parental melts. Supercritical alkali-rich brines are thought to produce the intense fenititization of country rocks associated with alkali-poor intrusive carbonatite bodies. Thus, quantitative data on the fluid-melt element partitioning is needed to evaluate the effect of fluid separation on the composition of carbonatitic magmas.
Experimental studies of element distribution in carbonate - water systems are hampered by the fact that neither fluid nor melt can be quenched isochemically in conventional high-pressure vessels. The reequilibration rates during cooling are very high and the composition of run products does not represent that of the phases at high pressures and temperatures. In order to overcome the quenching problems we used a double-capsule technique which allows us to separate the fluid and liquid phases during and after the runs. The inner platinum capsules were charged with carbonate mixtures and put inside the outer capsules charged with distilled water and diamond powder. The latter was used as an inert trap for the solids precipitating from the fluid phase on quenching. Carbonate melt and water fluid equilibrated through a small hole left in the upper end of the inner capsule. The starting charges for inner capsules were prepared from chemical-grade carbonates (CaCO3, MgCO3, Na2CO3 and K2CO3). The runs were performed in rapid-quench cold-seal pressure vessels at 1-2 kbar and 700 - 900°C in the two-phase (fluid + melt) stability region. The run products were dissolved in dilute HCl and analysed by ICP-AES.
Preliminary data on the CaCO3 - MgCO3 - Na2CO3
- H2O system show that fluid/melt partition coefficients for
Ca and Mg are similar and several times smaller than those of Na (Fig.
3.5-8). As the temperature increases from 700 to 900°C the partition
coefficient (D) for
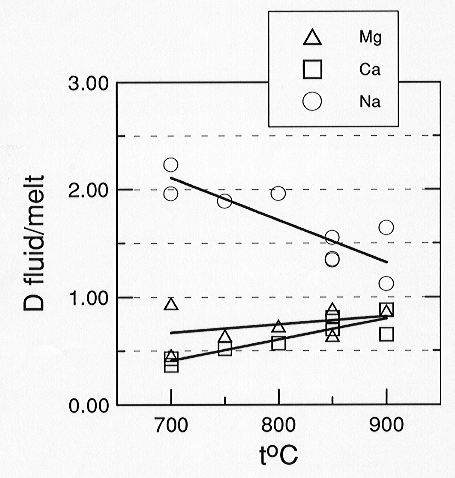 |
Fig. 3.5-8: Aqueous fluid / carbonate melt partition coefficients. Partition coefficients are calculated on an anhydrous basis. If the water contents of the two phases were considered, all numbers would be reduced by about one order of magnitude. |
Na (calculated on anhydrous basis) decreases from 2 to 1.3, while D for Ca and Mg increase from 0.4 to 0.9. Rough estimates of carbonate solubility in the fluid give 12 wt% for Na2CO3 and about 3-5 wt% for CaCO3 and MgCO3 at 700°C and 1 kbar pressure. The preliminary data imply that separation of aqueous fluid from carbonatitic magma at low pressures and temperatures may strongly affect the bulk composition of the melt.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page