

Next to olivine, pyroxenes are the most important constituents of the upper mantle. Even traces of water dissolved as OH defects in the pyroxene structure could represent a major fraction of the global water reservoir. Moreover, subducted oceanic crust is mostly made up of garnet and omphacite. If there were a significant solubility of water in either one of these phases, water could be recycled back into the mantle beyond the stability limit of hydrous phases.
Investigating water solubility in pyroxenes is particularly difficult due to the numerous solid solution series and trace element substitutions possible. It has been suggested that the incorporation of water may be strongly coupled with chemical defects. This would imply that water solubility would be largely controlled by the major and trace element chemistry of the host pyroxene. Moreover, saturating a natural pyroxene crystal with water may not yield thermodynamically meaningful solubilities unless the concentration of all chemical defects can be readjusted during the experiments.
In order to understand what really controls water solubility in pyroxene, we are currently conducting three series of experiments with MgSiO3 enstatite. These experimental series involve:
- Growing enstatite crystals under controlled P,T and water fugacity from high purity oxide starting materials.
- Similar experiments with starting materials doped with known amounts of major and trace elements.
- Annealing experiments of natural, gem-quality enstatite crystals at elevated water fugacity.
All spectra were taken on clear, inclusion-free single crystals several
10 to 100 µm in size. The spectra are very similar
to those of natural orthopyroxenes. The only difference is that the major
bands are shifted to somewhat lower frequencies than usually observed in
natural samples, which can be attributed to the lack of Fe substituting
for Mg. Since the interatomic distances in pure Mg-enstatite are shorter
than in Fe-bearing samples, hydrogen bonding will be stronger. This is
well known to reduce OH absorption band frequencies. The major conclusion
that can be drawn from the data in Fig. 3.5-9 is therefore that the OH
substitution in natural pyroxenes is not necessarily coupled to chemical
defects. The intrinsic solubility of water in very pure MgSiO3
enstatite reaches about 1000 ppm by weight at 100 kbars, a value comparable
to that measured on natural San Carlos olivine.
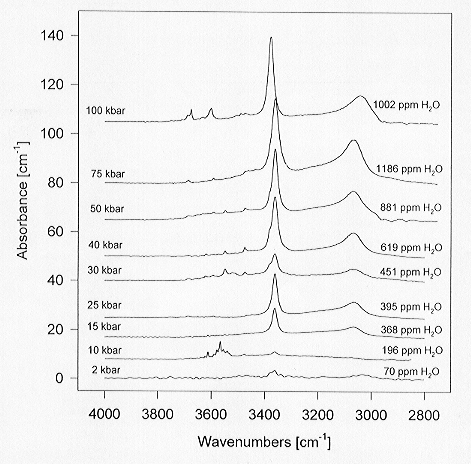 |
Fig. 3.5-9: Representative infrared spectra of pure MgSiO3 enstatite grown at 1100°C under water-saturated conditions from Mg(OH)2-SiO2 mixtures. Unpolarized spectra of clear, inclusion-free single crystals, are shown. |
In a series of synthesis experiments at 900°C we observed that the IR spectra of enstatite showed additional, very sharp OH bands around 3600 cm-1. These bands appear to be due to amphibole lamellae in the pyroxene structure, formed outside of the phase stability field of amphibole. These lamellae, although only present in tiny quantities, considerably increase the bulk water solubility in enstatite. Accordingly, the formation of amphibole lamellae is a viable mechanism to retain water in subducted crust beyond the breakdown of hydrous phases. Additional work is required to determine the stability limit of these lamellae and the thermodynamics of their formation. Infrared spectroscopy is a particularly useful tool for such a study, since it allows the detection of tiny quantities of amphibole solid solution inside pyroxene crystals.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page