

X-ray absorption spectroscopy has developed into a powerful technique for characterizing the environments of elements in both crystalline and amorphous systems. In particular, Al shows, due to its electronic structure, a very sensitive dependence of the X-ray absorption edge structure (XANES) features on coordination number and site distortion. The variation in the structure of aluminosilicate melts as a function of composition is very important in that it has measurable effects on their macroscopic and thermodynamic properties. In spite of the large number of studies of physical and chemical properties in this system, little information exists concerning their structural characterization. In view of the lack of structural data and in an attempt to derive possible correlations between the geometrical configuration of the silicate framework and the observed variations in the physical and chemical properties, we have engaged in a study devoted to the determination of the structure of glasses in the system M2O (MO)- Al2O3- SiO2, where M is an alkaline or alkaline earth cation, by X-ray absorption spectroscopy.
XANES spectra have been acquired at the SSRL (Stanford, California),
with the SPEAR storage ring operating at energy of 3 GeV and injection
current of about 100 mA. The monochromator was a double-crystal YB66 cut
along the (400) plane. Spectra were recorded in the total yield mode. Figure
3.6-11 shows the compositional range investigated for the system CaO- Al2O3-
SiO2.
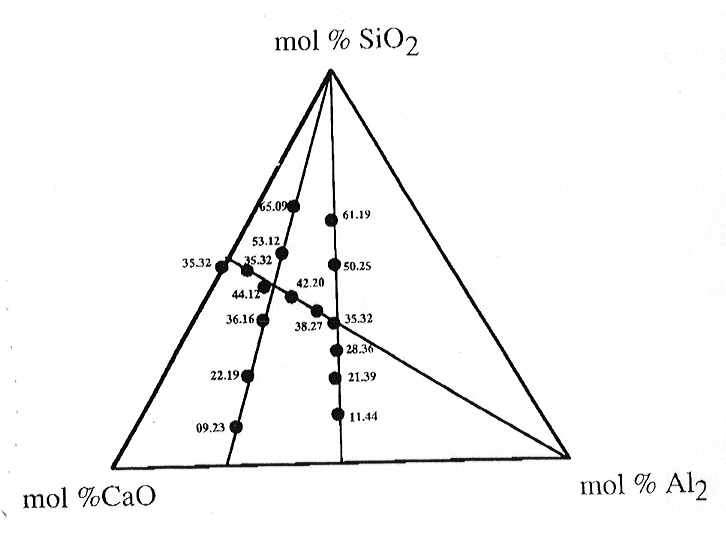 |
Fig. 3.6-11: CaO-Al2O3-SiO2 ternary diagram indicating compositions of glasses investigated by XANES spectroscopy. |
Al K-edge absorption spectra of representative samples in the same system
with different Al/Si are illustrated in Fig. 3.6-12. The K-edge spectra
are characterized by a main absorption band composed of two peaks of variable
intensity at about 1567 eV and 1570 eV. The energy positions of these two
peaks do not shift significantly as the composition changes whereas their
relative intensities exhibit a distinct variation, with the 1567 eV peak
decreasing and the 1579 eV peak increasing as the silica content decreases
(SiO2 varies from 65 mol% in the silica rich member to 9 mol%).
A broad peak at about 1585 eV is also present but its position and relative
intensity seem insensitive to compositional variations. The features observed
in these spectra are indicative of a strong compositional effect on the
structural environment of the aluminum in the silicate framework. The same
trend has also been observed for both peralkaline and peraluminous compositions
along the CaO-Al2O3-SiO2 system.
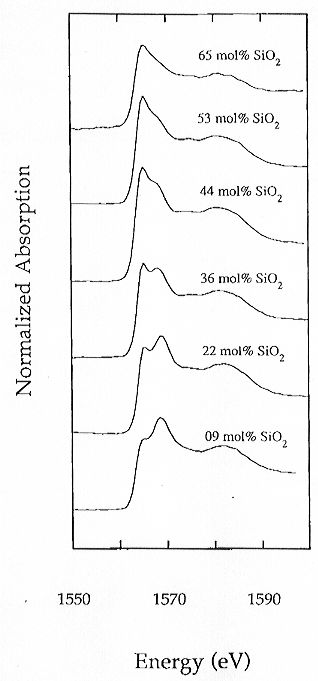 |
Fig. 3.6-12: Experimental XANES spectra of Al K-edge along the join SiO2-Ca3Al2O6. The relative intensity of the two peaks at about 1567 eV and 1570 eV changes as a function of composition. |
Cation (Na, K, Mg) substitution in the structure was also investigated, and preliminary results show that the effect on the structure due to cation substitution decreases in the order Ca>Mg>K>Na possibly in relation to the ionic potential of the observed cations. ELNES measurements have also been performed on the same samples in order to verify the existence of the observed features on a submicroscopic scale and to test for the possible presence of microcrystals. The perfect agreement between the two sets of spectra confirm the amorphous state of the material investigated.
Several possible hypotheses have been suggested to explain these features (coordination changes, distortion of tetrahedra, and TOT angle variations) and their variations as a function of composition. In order to differentiate among them and to better quantify the relationship between structure and chemical composition, we are currently performing XANES simulations according to the one-electron multiple scattering (MS) theory utilizing muffin-tin potentials with X-alpha exchange terms. Additional calculations investigating the effect of changes in the intertetrahedral angle and cation substitution (Na, K, Mg) are also planned for the near future.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page