

Characterization of the behaviour of titanium in silicate melts is of extreme importance both in the glass industry and in igneous petrology because of the very strong effect of even very small amounts of titanium on the physical and chemical properties of glasses. Given the relationship between structure and properties of silicate melts, an intensive effort has been taken to characterize the structure of Ti-bearing silicate glasses in order to shed light into the interpretation of the variation on physical and chemical properties in both natural and synthetic systems. In this project, we decided to investigate the structure of glasses in the M2O (MO)-Al2O3-TiO2 system, where M is an alkaline or an alkaline earth cation, by using XANES spectroscopy. Here we report results obtained for the system K2O-Al2O3-TiO2-SiO2. Preliminary results on the system BaO-Al2O3-TiO2 have also been obtained but they will not be discussed here. Glass samples have been synthesized by quenching in air from high temperature fusions. The samples investigated represent the addition of Al2O3 to a base of composition K2TiSi4O11 in amounts corresponding to mol. p.f.u. = 0.25, 0.50, 0.75, 1.00, 1.25, 1.50. This range of alkali/aluminum ratios crosses the leucite stoichiometry at 1.0. The Ti K-edge XANES spectra were measured at LURE (France) using the XAS2 line equipped with a Si (111) monochromator. Experiments at the Al and Si K-edge were carried out at SSRL (Stanford, California) with the SPEAR storage ring operating at energy 3 GeV and injection current of 100 mA. We operated on beam line SBO3-3 equipped with JUMBO monochromator of the double crystal type, which were two plates of a YB66 crystal cut along the (400) plane.
The XANES spectra exhibit different trends with increasing Al2O3
content. The aluminum and silicon K-edges do not seem to show any visible
changes as a function of Al content. Spectra recorded on the Ti K-edge
show, however, a distinct variation of the spectral features (pre-edge
peak P, peak A and peak B) as a function of increasing Al content, suggesting
a change in local geometry around the Ti absorber (Fig. 3.6-13). With increasing
Al2O3 content, peak P increases in intensity and
shifts to lower energy, peak A decreases in intensity with a minimum at
Al2O3 = 0.75% and then increases again slightly,
peak B clearly shifts toward higher energy (+ 5 eV). As demonstrated in
our previous work, the pre-edge peak intensity is correlated with titanium
coordination number in glasses. Variations of XANES features as a function
of aluminum content can be interpreted therefore to suggest a variation
of polyhedral geometry around titanium. To infer the average coordination
number of titanium on our glasses we applied the correlation curve between
the peak intensity and coordination number as calculated in our previous
studies. From this analysis, we calculated that the average CN increases
from the Al-free tetrasilicate composition up to 0.75 mol p.f.u. whereas
further addition of Al2O3, up to 1.25 mol p.f.u.,
decreases the average CN. These results can be interpreted as to indicate
a competition between different titanate complexes in 4-, 5-, and 6-coordination
as a function of chemical composition in the system.
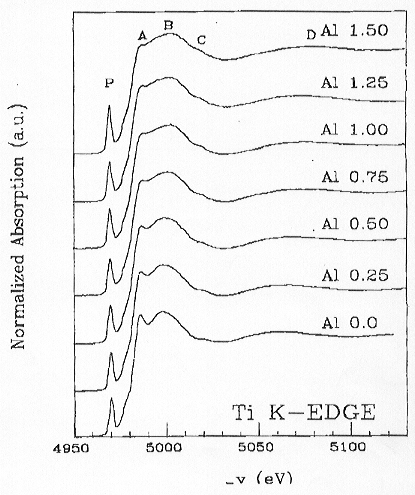 |
Fig. 3.6-13: Experimental XANES spectra of Ti K-edge in K2O-Al2O3-TiO2-SiO2 glasses varying in Al2O3 content. |
We performed 1 atm density measurements on the same samples in order
to detect any correlations between physical properties and structure in
these systems. These results are shown in Fig. 3.6-14. The density decreases
for the peralkaline composition and reaches a minimum for the composition
where Al2O3/K2O=1 and then increases again
for peraluminous samples. Several possible explanations can be envisaged
to interpret both the XANES and density data. The density minimum could
result from the combined effects of aluminum substitution for potassium
together with an increase in the stabilization of five-coordinated Ti polyhedra.
XANES simulations utilizing one-electron multiple scattering (MS) theory
to simulate the local Ti environment in a glass as well as additional measurements
to investigate the effect of different cations in the structure will be
carried out in the near future.
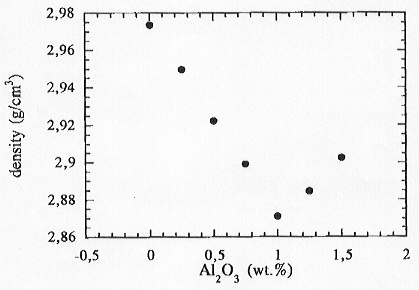 |
Fig. 3.6-14: Densities of K2O-Al2O3-TiO2-SiO2 glasses and their variation as a function of Al2O3 content. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page