

In the last decade spectroscopic studies and physical property measurements have been used to constrain the structural role of P in silicate melts and glasses. However, there are significant differences between many of the models proposed in the literature, and the solution mechanism of P remains the source of some controversy. Following from results previously presented in the 1995 Annual Report new 1 and 2-D 31P MAS NMR spectra have been obtained for glasses containing up to 7 mol% P2O5 in the system xNa2O-(1-x)Al2O3-2SiO2-yP2O5 (x =1.0 to 0.44). Our recent investigations have focused on, a) the influence and removal of water from peralkaline glasses, and b) making an unambiguous peak assignment, which is also consistent with previous spectroscopic and physical property measurements.
1H MAS and 1H-31P cross polarization experiments showed that the samples investigated previously contained proton bearing species (molecular water, OH-groups), whose presence was also confirmed by IR spectroscopy. Therefore, very peralkaline glasses were remelted under a flow of argon to remove water. Subsequent NMR spectra of remelted samples show that the peak at 9 ppm (identified as Na2HPO4) was no longer present.
In the Al-free glasses only two resonances occur, at +13 and +2 ppm.
These chemical shifts are similar to those of crystalline Na3PO4
and Na4P2O7 monomers and dimers respectively,
and thus are assigned to these species. In aluminium-bearing peralkaline
glasses an additional well defined resonance is observed at +7 ppm, as
well as a broad resonance occurring at -5 ppm which, in the light of suggestions
in the literature, we tentatively assigned to end- and mid-groups of longer
phosphate chains. However, this interpretation is somewhat ambiguous, and
thus it was decided to perform a two dimensional 31P spin diffusion
experiment on a glass containing all four of the resonances described above.
The spectrum (Fig. 3.6-15) shows off-diagonal peaks for the resonances
of monomer and dimer units, but no off-diagonal peaks for the resonance
at -5 ppm. Such a spectrum is clearly not compatible with the idea of longer
chains, where one would expect considerable off-diagonal intensity between
mid- and end-groups.
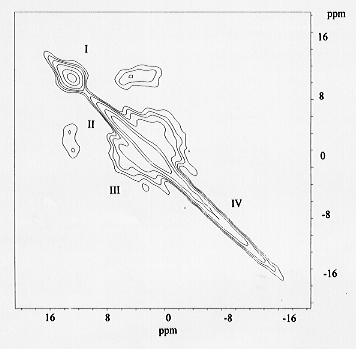 |
Fig. 3.6-15: 31P spin-diffusion MAS NMR spectrum of a glass of composition 0.7Na2O-0.3Al2O3-2SiO2-0.12P2O5. I and III denote the signals of phosphate monomers and dimers respectively. Resonances II and IV are assigned to PO4 monomers associated with 1 and 2 Al. |
In consequence we propose a new model for the solution mechanism of phosphorus in which the resonances at +7 ppm amd -5 ppm are assigned to monomer units with one and two Na replaced by Al, respectively. Such environments have been described for crystalline compounds by Dollase et al, who showed that the chemical shifts of these environments are +6 and -5 ppm, respectively. Such phosphate monomers which are attached to the aluminosilicate network through association with Al would be expected to be protected from other P environments, as suggested by the 2-D spectrum. Values of the average Na/P ratio of the sodium aluminophosphate complexes estimated from these NMR spectra are shown to be consistent with values estimated from recent viscosity measurements on the same compositions. For metaluminous glasses our spectra do not imply the presence of longer phosphate chains as proposed by Gan and Hess, but are consistent with the solution of P as a phosphate monomer with all three available oxygens attached to aluminium in the network. In this model P may be added to fully polymerised aluminosilicates without the need to break apart stable tetrahedral aluminate complexes. In peraluminous glasses it is inferred that P interacts with excess aluminium present as triclusters to form AlPO4 complexes.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page