

Olivine is the dominant phase in the Earth's upper mantle and therefore controls many physical (e.g. creep) and chemical processes (e.g. partial melting). Knowing the relevant physico-chemical properties of olivine is essential in quantitatively modelling these processes. One key property is diffusion because it describes how fast the composition reequilibrates in response to changing the thermodynamic state of a system. Thus knowing diffusion coefficients as a function of pressure, temperature and oxygen fugacity enables the timescale of geochemical processes to be assessed.
Because of the importance of quantifying diffusion there exists an extensive database for olivine diffusion coefficients at 1 bar and high temperatures. However there have been only a few studies of diffusion in olivine at high pressures (up to 9 GPa). In this study we examined the pressure dependence of Mg-Fe exchange and Mn and Ni diffusion up to pressures of 12 GPa at 1400°C. This enables us to determine the pressure dependence of chemical diffusion of major and trace elements to the upper pressure-limit of olivine stability.
To deduce the pure pressure effect we tried to fix all other relevant parameters (P, T, fO2) by performing isothermal high pressure experiments with the same sample geometry in the same chemical environment. The samples were prepared as a diffusion couple consisting of two olivine single crystals enclosed in a thick-walled gold capsule. Because gold is a relatively soft and ductile material it aids in achieving hydrostatic stress conditions and also the solubility of Fe in Au is low ( < 0.3 wt% at our experimental conditions). For all experiments we used a 500 t Walker type multi-anvil apparatus with constant temperature of 1400°C and 24 hour run times. Temperature was automatically controlled and remained constant at the thermo couple within 1°C.
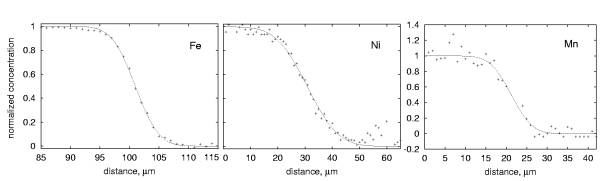
We used samples prepared from the same two crystals in all our experiments which have been oriented using the Laue X-ray diffraction technique so that diffusion occurred along the crystallographic c-direction. One of the crystals was synthetic forsterite and the other one San Carlos olivine with Mg/(Mg+Fe) = 0.94. Diffusion profiles have been measured with an electron microprobe and are 10 to 20 µm long. By having a relatively small difference in Mg-content we avoid large structural misfits across the interface, which potentially could lead to high dislocation densities.
All diffusion profiles are symmetric and can be fitted with a simple error-function solution of the
diffusion equation with infinite boundary conditions (Fig. 3.1-9). In Fig.
3.1-10 the diffusion coefficients are shown as a function of pressure.
To check for reproducibility we repeated the experiments at 10, 11, 11.5
and 12 GPa. Errors are typically half an order of magnitude or less depending
on the concentration of the species measured. For all cations we observe
a relatively weak dependence of diffusivity on pressure and activation
volumes are close to zero.
 |
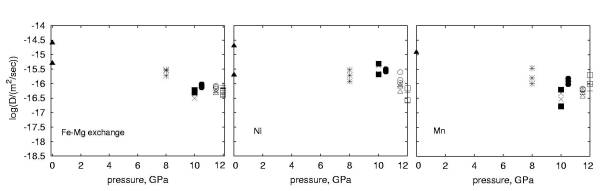 |
In order to compare these data with results of studies at 1 bar, the oxygen fugacity must be known. Based on a previous study where diffusion experiments were also done using Au capsules the fO2 is estimated to be 10-8 to 10-9 bar. However we estimate that the effect of a change in oxygen fugacity of 3 orders of magnitude results in a change in the diffusion coefficient of at most 1 order of magnitude, which is only twice the uncertainties in our measurements. The comparison with the 1 bar data shows an overall decrease of diffusivity of the order of one log unit in all cases. If the relatively large activation volume of 5.5 cm3/mole reported in the literature for pressures between 1 bar and 3.5 GPa is correct, then this would mean a decrease of the activation volume with increasing pressure. As a next step we intend to perform experiments in Ni-capsules with added NiO in order to constrain better the oxygen fugacity.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page