

Experiments on chondritic compositions indicate that Ca-rich silicate perovskite is, together with Mg-rich silicate perovskite and magnesiowüstite, an important constituent of the lower mantle and may reach concentrations up to 10%. The physical and chemical properties of silicate perovskites can be influenced by the substitution of trivalent cations R3+ (such as Al, Fe, Cr) in the crystal structure by different mechanisms. One possibility is the replacement of 2 Si4+ by 2 R3+, which creates one oxygen vacancy. Whereas the incorporation of trivalent cations by the oxygen defect mechanism seems to be strictly limited to a few percent in Mg-rich silicate perovskites (cf. Annual Report 1999), the Ca-rich silicate perovskite might show a complete solid solution with CaFeO2.5 or the formation of discrete intermediate phases as in the system CaTiO3 - CaFeO2.5. The latter system shows order-disorder of cations and oxygen defects which causes appreciable changes in physical properties such as the electrical conductivity (cf. Annual Report 1999). As in the Mg-rich silicate perovskites where previous studies have shown significant changes in the elastic and transport properties upon the substitution of minor amounts of R3+, the amount of Fe3+ in Ca-rich silicate perovskite could have significant implications for the interpretation of geophysical and geochemical data.
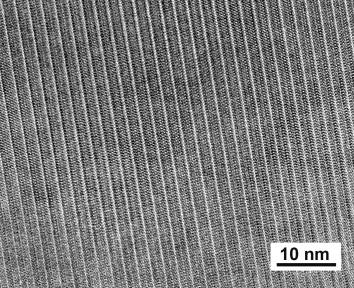
In initial experiments we synthesized a perovskite of the composition near CaFe0.4Si0.6O2.8 by loading powdered mixed oxides into a Re capsule and running at temperatures between 1400°C and 1600°C and pressures of 16 GPa using a multianvil press. The presence of Fe3+ stabilizes CaSiO3 perovskites such that the crystal structure is preserved during quenching and this phase is relatively stable under the electron beam, unlike the endmember CaSiO3 which becomes amorphous upon decompression. Mössbauer spectroscopy shows that all iron is trivalent and occurs in octahedral coordination. Electron energy loss spectroscopy (EELS) at the Si L32, Si K and Fe L32 edges confirms the oxidation state of iron and indicates that Si appears in both octahedral and tetrahedral coordination. Electron diffraction and HRTEM images show a strong periodicity of 2.17 nm (cf. Fig. 3.2-1), possibly related to ordering of oxygen defects. Bright field images demonstrate the existence of polysynthetic twinning on a scale of approximately 500 nm, which might be due to a phase change upon cooling.
 |
| |
These results confirm that large amounts of Fe3+ can be incorporated into CaSiO3 perovskite by a defect mechanism resulting in oxygen vacancies. Our results suggest that lower mantle Ca-rich silicate perovskite incorporates iron almost exclusively in the trivalent form and could provide a sink for Fe3+, depending on the local chemistry. Further experiments with different compositions on this join are planned to investigate the interrelations between the bulk chemistry and the structural as well as the physical properties of Ca-rich perovskites.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page