

Pyroxenes are common minerals in the Earth's crust and upper mantle, and they exhibit a complex polymorphism involving both reconstructive phase transitions between orthopyroxene and clinopyroxene, and non-quenchable displacive phase transitions between the three pyroxene polymorphs (see Annual Report 1998). Understanding the chemical controls on the stability fields of the various pyroxene polymorphs can yield insights into the pressures and temperatures experienced by high-pressure rocks containing pyroxenes. One such occurence is the "Alpe Arami" peridotite, a high-pressure rock found in the Italian Alps. Following the apparent identification of high-pressure mineral assemblages in this peridotite, controversy has recently raged over whether the current mineral assemblages really preserve an indication of a very-high-pressure origin, perhaps as deep as >300 km. Such an interpretation would have profound consequences for our understanding of the mechanisms of exhumation of high-pressure terraines and their preservation.
The low-clinopyroxene found in the Alpe Arami peridotite is a "pigeonite", chemically speaking mostly a solid solution between enstatite (MgSiO3) and ferrosilite (FeSiO3) with Ca as the major additional component. The pigeonite as thin lamellae within a matrix of orthopyroxene, a microstructure that suggests that the pyroxene mineral grains were originally a single phase, and that the clinopyroxene exsolved from the orthopyroxene as the peridotite unit cooled down from high temperatures during its exhumation. The additional observation that the pigeonite contains anti-phase domains suggests two possible histories for the pyroxenes. The anti-phase domains may have formed during exsolution and growth of low-clinopyroxene within the orthopyroxene stability field, in which case no strong constraints can be placed on either the temperature or the pressure at which this occurred. Alternatively the orthopyroxene may have exsolved a high-clinopyroxene phase at very high pressures and temperatures, and the exsolved phase later inverted to pigeonite. If the latter is the case, then the pressure of the inversion between the high and low clinopyroxenes would place a minimum limit on the pressure at which the exsolution occurred, and thus an estimate of the depth from which the peridotite has been exhumed.
The phase transition between low-clinopyroxene and high-pressure (HP-)clinopyroxene can be observed when single-crystals are compressed in the diamond-anvil cell at room pressure. Because the transition pressure increases with temperature, the room pressure measurements put a minimum bound on the transition pressure in the rock during exhumation. Over the past several years our single-crystal x-ray diffraction (e.g. Annual Reports 1998, 1999) and spectroscopic studies of the behaviour of clinopyroxenes have shown that the room-temperature transition pressure decreases with increasing size of the cations such as Mg, Fe, Mn within the clinopyroxene structure. In addition, we had shown that the transition pressure decreases more strongly when cations such as Fe2+ or Cr3+ are present, presumably because of crystal-field stabilization effects.
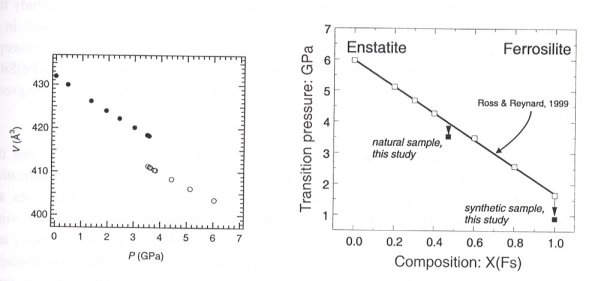
Thus, an outstanding question for mantle clinopyroxenes is: what is the effect of Ca substitution in Fe-containing clinopyroxenes? The transition pressure may decrease due to the larger ionic radius of Ca, or this may be compensated by a decrease in the crystal field stabilization by Fe. We have therefore studied a natural and a synthetic sample with small amounts of Ca and bulk compositions close to the enstatite - ferrosilite join by high-pressure single-crystal X-ray diffraction to determine their transition pressures (e.g. Fig. 3.2-3). Initial results indicate that Ca substitution into Mg-Fe clinopyroxenes results in a decrease in transition pressure relative to Ca-free clinopyroxenes with the same Fe/Mg ratio (Fig. 3.2-4).
 |
||
By using known systematics of the phase diagram with composition, we conclude that the minimum depth required for formation of HP-clinopyroxene in the Alpe Arami peridotite as a stable phase is circa 200 km, considerably lower than first estimated.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page