

Diamond is known for its outstanding properties such as highest hardness and heat conductivity and chemical inertness; its thermal stability is however limited. Due to the structural similarities of diamond (a=3.567 Å) and cubic BN (a=3.617 Å), mixed cubic B-C-N compounds have been synthesized at high pressures and temperatures. These ternary compounds may display similar physical properties as diamond but have higher thermal stabilities, opening a field of applications. We have employed shock and diamond anvil (at 3000 K and 31.6 GPa) techniques for synthesis using graphitic BC2N as a starting material. Analytical transmission electron microscopy (ATEM) combined with electron energy loss spectroscopy (EELS) was used to study the micro- and electronic structure of the B-C-N compounds.
Both shock and diamond anvil techniques result in the formation of nanocrystalline B-C-N aggregates with mean grain sizes on the order of 5-10 and 20-30 nm, respectively. In particular the shock-synthesized sample shows immediate damage under the electron beam. The electron diffraction ring patterns are fully consistent with cubic B-C-N compounds, having d-spacings that are indistinguishable from those of diamond. A superstructure was not observed, suggesting that B and N are randomly distributed in the crystal structure.
The compositions of B-C-N compounds were quantified on the basis of B K, C K, and N K ELNES spectra using calculated partial ionization cross sections (Fig. 3.2-6). The shock-
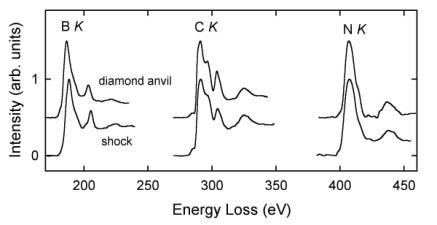 |
synthesized sample maintained the BC2N composition of the precursor material, whereas the sample synthesized in a diamond anvil apparatus consists of three decomposition products: cubic BN, BC12N, and subordinate diamond. The high carbon content in the latter B-C-N compound is also reflected in the sharp C K ELNES spectrum, which is almost identical to that of diamond. The B K and N K ELNES of both B-C-N compounds are similar to those of cubic BN but less sharp, indicating local disorder around excited B and N atoms in the structure. This study demonstrates that the solubility of B and N in diamond is coupled and distinctly depends on synthesis conditions. The duration of pressurization seems to be a key factor and high temperatures favor a decomposition of B-C-N compounds.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page