

Experimental metal-silicate partitioning data for highly siderophile elements (HSEs) such as Os are particularly useful in discriminating between different processes that may have occurred during the accretion of the Earth. This has motivated many studies in recent years to study HSE solubility in silicate melt as a function of pressure, temperature, oxygen fugacity and melt composition. However, in many previous studies at low oxygen fugacities, micro- to nanometer size HSE metal particles have been present in the silicate melt, which have drastically contaminated the silicate glass analyses. This has led to a large scatter in the reported HSE solubility data at low oxygen fugacity. The purpose of the present work was to re-investigate the dependence of Os solubility on oxygen fugacity using the stirred crucible technique, a technique that might help in minimizing this so-called "micronugget" contamination problem.
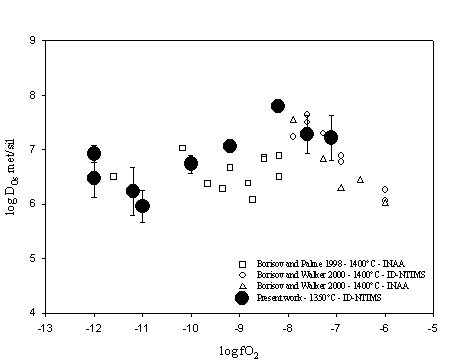
Experiments were performed at one bar and 1350°C and are described in the1998 Annual Report. Samples were analysed by electron microprobe for major elements at the Bayerisches Geoinstitut and by Isotopic Dilution Negative Thermal Ionisation Mass Spectrometry (ID-NTIMS) at IPG of Paris. Experimental results on the effect of oxygen fugacity on Os partitioning between metal and anorthite-diopside eutectic composition melt are presented in Figure 3.3-4. The experimental data from Borisov and Palme (1998) and Borisov and Walter (2000) are also presented for comparison.
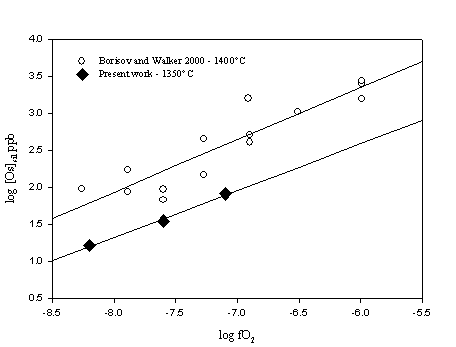
For high oxygen fugacities (log fO2 > -8) the Os solubility data imply a valence of +3.15 for Os in the melt. At low oxygen fugacities, the discrepancy in our data makes it difficult to determine the oxidation state of Os in the melt. Due to the data scatter we suspect that the bulk analyses of our glass samples may still be somewhat contaminated by the presence of micronuggets. Therefore, for low oxygen fugacities (log fO2 < -8), the solubilities of Os in the melt are most likely overestimated and the data do not reflect uniquely the effect of oxygen fugacity. Figure 3.3-5 shows the Os solubility data in the high oxygen fugacity range (log fO2 > -8) compared with those reported from a previous study.
 |
 |
A similar "micronugget" problem has also been observed in Re solubility experiments at low oxygen fugacities (see Annual Report 1999). These Re-bearing samples were first analysed using Instrumental Neutron Activation Analysis (INAA), which like ID-NTIMS is a bulk technique that samples the entire glass, and therefore "micronuggets" cannot be avoided. The development of the LA-ICP-MS microanalytical technique, however, made it possible to re-analyse accurately the Re content of these glasses using a small beam diameter to avoid the "micronugget" contamination. LA-ICP-MS analyses proved unsuccessful for Os-bearing glasses, however, as the concentrations are below the detection limits of this technique.
Although scatter in the Os partitioning data at low oxygen fugacities is still present over the entire range of oxygen fugacity investigated, Os metal-silicate partition coefficients are generally higher than those previously reported. This suggests that the glass samples produced in the present study are less contaminated by metal particles than in previous studies. The combination of the stirred crucible technique and ID-NTIMS analysis can therefore minimize the effects of "micronuggets" in the glass samples and provide improved Os solubility data at low oxygen fugacities.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page