

According to recent models of core formation, the current abundances of at least some of the siderophile elements in the Earth's mantle can be explained by equilibration between metal and silicate liquid at 25-30 GPa at the base of a deep magma ocean. According to such models, chemical interaction between liquid metal and crystalline silicates at greater depths in the lower mantle must be limited by kinetics. To constrain core formation models better (e.g. the mechanisms by which metal was transported through the lower mantle), we are quantifying the rates of equilibration between liquid metal and silicate liquid in a magma ocean and between liquid metal and crystalline lower-mantle minerals through experimental studies combined with kinetic modelling.
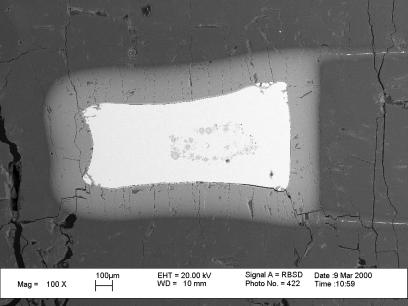
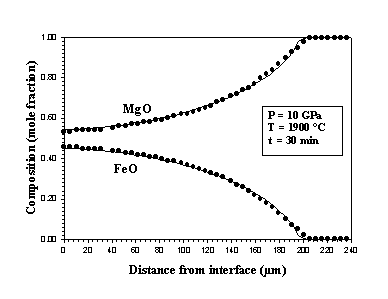
Reactions between liquid Fe-Ni metal and magnesiowüstite have been studied at 10 GPa and 1900°C for times up to 30 minutes using a multianvil apparatus (see also 1999 Annual Report). The liquid metal is contained in a capsule fabricated from an MgO single crystal that reacts during the experiments to form magnesiowüstite (Fig. 3.3-8).
Results obtained after reaction times ranging from 30 seconds to 30 minutes show that the rate of metal-oxide equilibration is controlled by Fe-Mg and Ni-Mg interdiffusion in magnesiowüstite. Equilibration also involves an interface reaction that consumes oxygen that is dissolved in the metal. The observed concentration profiles in magnesiowüstite have been fitted using the diffusion equation:
 |
where C is composition, D is the diffusion coefficient which may depend on composition, t is time, x is distance and v is the velocity of the metal-magnesiowüstite interface. In order to model Fe-Mg interdiffusion, we used the diffusion coefficient determined at one bar by Mackwell et al. (in preparation), with an activation volume term added to take into account the effect of pressure:
Here DFe-Mg is the interdiffusion coefficient which is strongly dependent on the FeO content
of the magnesiowüstite (![]() ),
),
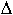 H is the activation enthalpy
of diffusion, P is pressure, T is temperature (K) and
H is the activation enthalpy
of diffusion, P is pressure, T is temperature (K) and 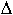 V
is the activation volume. For
V
is the activation volume. For 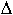 H we used
the value of 167 kJ mol-1 determined at 1 bar by Mackwell et
al. (in preparation). Concentration profiles can be fitted well with the
model using an activation volume of about 5 cm3 mol-1
(Fig. 3.3-9).
H we used
the value of 167 kJ mol-1 determined at 1 bar by Mackwell et
al. (in preparation). Concentration profiles can be fitted well with the
model using an activation volume of about 5 cm3 mol-1
(Fig. 3.3-9).
Similar experiments performed to study the kinetics of equilibration between liquid metal and MgSiO3 perovskite at 25 GPa have been less successful because of problems in synthesizing a sample capsule that consists of 100% perovskite. Therefore we are instead studying Fe-Mg interdiffusion in (Mg,Fe)SiO3 perovskite at 25 GPa using diffusion couples. Preliminary results obtained at 1800°C and 23 GPa for 8 hours indicates no interdiffusion on a scale detectable by the electron microprobe. We therefore estimate that Fe-Mg interdiffusion is slower in perovskite than in magnesiowüstite by at least 4 orders of magnitude at 1800°C.
 |
Extrapolating diffusion data to conditions deep in the early Earth makes it possible to estimate equilibration times between liquid metal and different mantle minerals. For crystals 5 mm in radius, equilibration times typically range from about one year for wadsleyite to 10-100 years for olivine and magnesiowüstite. The equilibration times for silicate perovskite may be several orders of magnitude larger. These estimates therefore suggest that the extent of equilibration between metal and silicate during core formation could vary greatly as a function of depth in the Earth. In addition, much of the metal in the core may never have fully equilibrated with the lower mantle even if the metal had segregated along grain boundaries.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page