

(Mg,Fe)(Si,Al)O3 perovskite and (Mg,Fe)O ferropericlase are believed to be the main components
of the lower mantle. Partitioning of Fe and Mg between these two phases
can affect lower mantle properties and dynamics, particularly since Fe2+
and Fe3+ are expected to behave differently in each phase. Previously
we showed that Fe3+/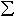 Fe in (Mg,Fe)(Si,Al)O3 perovskite
is a strong function of the Al concentration (Annual Report 1997). Until
recently, Fe/Mg partition coefficients could only be calculated in terms
of total iron, but a recent breakthrough has enabled both the Fe2+/Mg
and Fe3+/Mg partition coefficients to be calculated for (Mg,Fe)(Si,Al)O3
perovskite - (Mg,Fe)O ferropericlase assemblages using a combination of
Electron Energy Loss Spectroscopy (EELS) and the electron microprobe (see
Annual Report 1999).
Fe in (Mg,Fe)(Si,Al)O3 perovskite
is a strong function of the Al concentration (Annual Report 1997). Until
recently, Fe/Mg partition coefficients could only be calculated in terms
of total iron, but a recent breakthrough has enabled both the Fe2+/Mg
and Fe3+/Mg partition coefficients to be calculated for (Mg,Fe)(Si,Al)O3
perovskite - (Mg,Fe)O ferropericlase assemblages using a combination of
Electron Energy Loss Spectroscopy (EELS) and the electron microprobe (see
Annual Report 1999).
Synthetic mixtures of (Mg,Fe)(Si,Al)O3 perovskite and (Mg,Fe)O ferropericlase were synthesised from oxide starting
mixtures in Re capsules at 26 GPa and 1750°C using a multianvil press.
The run time was extended to 26 h to maximise chemical homogeneity of the
run product. Experiments produced well crystallised samples where major
element concentrations in both phases could be reliably determined using
the electron microprobe. Optical thin sections were prepared, then ion
thinned to electron transparency. EELS was used to determine Fe3+/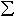 Fe
for each phase (Fig. 3.3-14), and combined with the major element concentrations
determined from the electron microprobe, could be used to determine Fe2+/Mg
and Fe3+/Mg partition coefficients for (Mg,Fe)(Si,Al)O3
perovskite - (Mg,Fe)O ferropericlase assemblages.
Fe
for each phase (Fig. 3.3-14), and combined with the major element concentrations
determined from the electron microprobe, could be used to determine Fe2+/Mg
and Fe3+/Mg partition coefficients for (Mg,Fe)(Si,Al)O3
perovskite - (Mg,Fe)O ferropericlase assemblages.
We determined the partition coefficient KD = (xFe-pv/xMg-fp)/ (xFe-fp/xMg-pv) for an assemblage containing Al to be nearly identical to the value for an Al-free assemblage if Fe2+ only is considered, but the partition coefficient is nearly a factor of two greater if Fe3+ only is considered (i.e. Fe3+ is strongly partitioned into the perovskite phase in the presence of Al). The combined effect of Fe2+ and Fe3+ partitioning is that the partition coefficient considering total Fe is slightly higher for the assemblage containing Al, but we note that overall the partition coefficients are significantly less than one, which means that total Fe is partitioned into (Mg,Fe)O more strangly than (Mg,Fe)(Si,Al)O3 perovskite even in the presence of Al.
Experiments are underway to investigate the effects of temperature, iron composition and the presence of minor elements such as Cr and Na.
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page