

The partitioning of Fe between mantle minerals will have a large effect on the position and width of seismic discontinuities and the oxygen fugacity of the mantle. At pressures where olivine dominates the mineralogy of the mantle, the low solubility of ferric Fe in this phase will result in a relatively high oxygen fugacity (FMQ±2) as recorded in mantle xenoliths. At higher pressures, however, the increased solubility of Fe3+ in dominant mantle minerals such as wadsleyite, ringwoodite and majorite garnet will result in a lowering of the ambient oxygen fugacity, possibly to values close to the IW buffer. We have performed experiments to investigate the effect of oxygen fugacity on the partitioning of Fe between mantle minerals and in addition we have measured the partitioning of Fe3+ between these phases.
Multianvil experiments were carried out between 17 and 26 GPa using a 7/3 assembly and multi-chamber capsules that allowed many samples to be run in a single experiment (see Annual Report 1999). By using Fe and Re sample capsules, the oxygen fugacity in the experiments could be varied. Since magnesiowüstite is stable over a large pressure range, it was used as a standard material to determine Fe-Mg partitioning properties between many silicate phases. By combining silicate-magnesiowüstite partitioning data for different silicate phases, silicate-silicate partition coefficients can be calculated along with thermodynamic mixing properties for the silicate phase using the known mixing properties of the oxide.
Figure 3.3-16a shows results of partitioning experiments between ringwoodite and magnesiowüstite using Re capsules. A regression line is also shown for experiments performed in Fe capsules (reported in 1999 Annual Report). A significant difference can be seen between the results from Re and Fe capsules due to the difference in oxygen fugacity imposed by each capsule material. EELS measurements allow the partitioning of Fe3+ between phases to be determined as shown in Figure 3.3-16b. Measurements on a single wadsleyite sample showed that significant Fe3+ variation can occur, which may reflect disequilibrum with the Fe capsule wall even after a run duration of 10 hours at 1400oC. As a consequence, in later experiments powdered metallic Fe was also added throughout the samples. While Fe3+ concentrations in Fe capsules are low for both ringwoodite and magnesiowüstite, in Re capsules magnesiowüsitite contains significantly more Fe3+ than ringwoodite. The results of these experiments have been used to determine true Fe2+-Mg2+ activity coefficients for high pressure mantle minerals and will allow the effect of oxygen fugacity on transformations responsible for seismic discontinuities to be assessed.
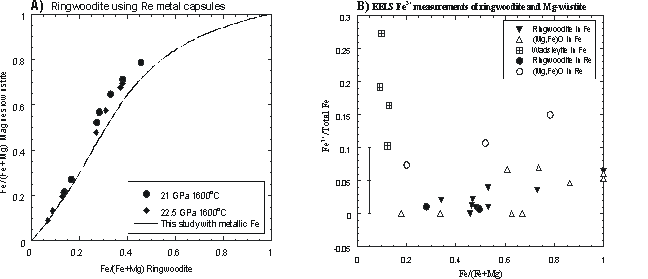 |
 Fe-Mg partitioning of using Re
and Fe capsules. Deviation from the Fe-saturated experimental results occurs
when Re capsules are used due to the increase in Fe3+ concentration.
B) EELS measurements reveal that while Fe3+ concentrations are
relatively low in ringwoodite and magnesiowüstite produced at Fe saturation,
in Re capsules the Fe3+ content of magnesiowüstite is increased
significantly. Fe-Mg partitioning of using Re
and Fe capsules. Deviation from the Fe-saturated experimental results occurs
when Re capsules are used due to the increase in Fe3+ concentration.
B) EELS measurements reveal that while Fe3+ concentrations are
relatively low in ringwoodite and magnesiowüstite produced at Fe saturation,
in Re capsules the Fe3+ content of magnesiowüstite is increased
significantly. |
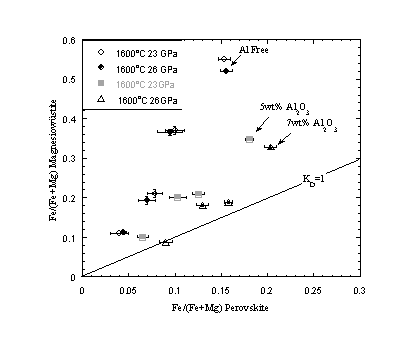
Partitioning of Fe between magnesiowüstite and silicate perovskite has also been measured in the presence and absence of Al2O3 using Re capsules. As with previous experiments equilibrium compositions are approached from starting compositions on both the Fe-rich and Fe-poor sides. Such reversals revealed that run times of at least 10 hours at 1600oC are required for the perovskite and oxide compositions to approach equilibrium. Many previous studies have performed experiments for much shorter run times, which may explain the large data scatter in the literature on this subject. In Al-free compositions Fe is preferentially partitioned into magnesiowüstite, in the ratio of approximately 1:3. For Al-bearing experiments, starting compositions contained 8 wt.% Al2O3, which resulted in the formation of aluminous perovskite and garnet. Figure 3.3-17 shows that as the partitioning of Al2O3 into perovskite increases with pressure, Fe partitions more equally between perovskite and magnesiowüstite and at high pressure approaches a 1:1 distribution. Partitioning data collected for high Al-contents do not extrapolate back to the origin, which means KD also becomes a strong function of the total Fe content. As most estimated mantle peridotite compositions have Al2O3 contents less than 5 wt.%, we concur with Lauterbach et al. (this Annual Report) that under lower mantle conditions magnesiowüstite is still likely to be the dominant Fe bearing mineral.
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page