

In a previous O K edge ELNES study we have used brucite to identify hydrogen-related modifications in the electronic structure of hydrous minerals (cf. Annual Report 1998). Here we focus on the kinetics and mechanism of dehydration of brucite, a representative structural component in a large number of hydrous, rock-forming minerals. Therefore, the results of this study may provide some basic information on the thermal mobilisation of water in metamorphic rocks.
Transmission electron microscopy (TEM) techniques were employed to study in-situ the electron-beam induced dehydration of brucite Mg(OH)2. Under the electron beam the hexagonal platelets of brucite immediately decompose and show a volume contraction, which first occurs in the rim and then affects the centre of the grains. Low-Loss EEL spectra reveal a simultaneous change in the local mass thickness of 50-55%. Combining the data on the morphological contraction and local mass thickness, it follows that the porosity in the dehydrated material is 37.5-50%. The porosity of the dehydrated material is hence only slightly smaller than the maximum theoretical porosity (54%). In agreement with HRTEM observations, this result suggests that the decomposition product is not only composed of numerous, tiny MgO crystallites but also contains many voids.
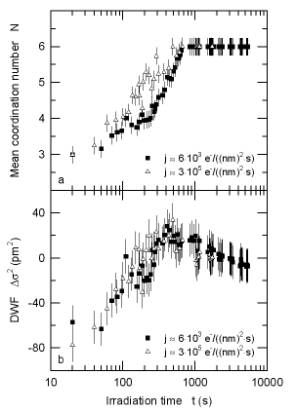
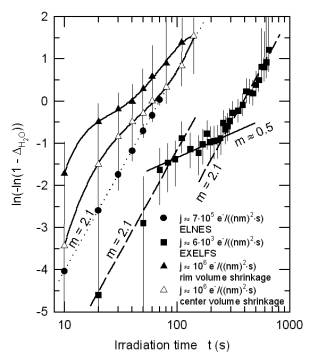
Information on the local environment of the oxygen atoms was derived from extended energy-loss fine structure (EXELFS). During dehydration the coordination number of oxygen shows the expected increase from 3 for brucite to 6 for MgO (Fig. 3.4-5a). In a transient state the Debye-Waller factor reaches a maximum (Fig. 3.4-5b), indicating a highly disordered intermediate state. These data allow us to model the water loss and to examine reaction kinetics applying the Avrami equation (Fig. 3.4-6). The decomposition of brucite is interpreted as a complex three-stage process: (i) It proceeds first via an interface-controlled process, starting at the rim of each brucite grain with water escaping along the basal planes. (ii) The dehydrated lattice then collapses at the rim, whereas the core region is still hydrated. To further dehydrate the grain, the voids have to interconnect and rearrange themselves in the form of a network which slows down the decomposition. At this stage, the process is diffusion-controlled. (iii) Finally, the pores become interconnected and reach the surface. The dehydration accelerates and is again an interface-controlled process.
 |
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page