

Experimental measurements of transport properties of liquid iron alloys provide important constraints on Earth's outer core properties. The chemical diffusivity of the outer core has been largely ignored, despite the fact that chemical buoyancy is one of the, and possibly the main, driving forces for outer core convection. This study has measured the self-diffusivity of liquid iron at high pressures in the multi-anvil press. Tracer diffusion experiments were performed, with foils of 57Fe diffusing into a sample of normal isotopic abundance. Once at pressure, temperature was increased at a constant ramp rate of 300 °C/s to experimental conditions and maintained for durations of 0 to 30 s. Precision in temperature control was better than 1 %. The diffusion profile in the recovered quenched sample was measured using the ion probe at Edinburgh University.
The measured 57Fe diffusion profiles were fitted according to the tracer equation:
C/C0 = exp-(x2/4Z) (1),
where C is the concentration at distance x from x0, C0 is the concentration at x0, and Z is the diffusivity, D, integrated over time, t:
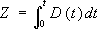 (2).
(2).
In experiments where ramping up to the desired temperature is a negligible proportion of the heating duration, D is constant, Z = Dt and (2) becomes the standard tracer equation. However, because diffusion in these experiments occurred while increasing temperature, the diffusivity varied with time. Experiments quenched from different temperatures during constant heating rate experiments thus result in different values of Z, everything else being constant. Experimentally determined values of Z were integrated assuming an Arrhenius dependence of D on temperature:
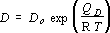 (3),
(3),
where T is a linear function of t, to give values of D at each experimental temperature. The pre-exponential, D0, and apparent activation energy, QD, were treated as parameters for fitting and errors were minimised by an iterative least squares process. Results obtained using this integration procedure were identical to those obtained from traditional "isothermal" series of experiments in which the effect of finite melting and quenching duration was evaluated by performing repeat experiments under identical temperature, ramp rate conditions and different durations.
The self diffusivity, D, can be well approximated by the following equation:
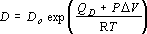 (4),
(4),
where D0 is the intercept of diffusivity at infinite temperature, QD
is the activation enthalpy and 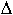 V
is the activation volume; D0 = 6.0±0.4x10-2
cm2s-1,
QD = 92.5±4
kJmol-1,
V
is the activation volume; D0 = 6.0±0.4x10-2
cm2s-1,
QD = 92.5±4
kJmol-1,  V = 2.9±0.3
cm3mol-1. The pressure dependence of diffusion in
liquid iron is considerably larger than predicted from theories of transport
in liquid metals, but there is excellent agreement between the present
results and recent experimental estimates of viscosity at the melting temperature
(grey line in 3.5-3).
V = 2.9±0.3
cm3mol-1. The pressure dependence of diffusion in
liquid iron is considerably larger than predicted from theories of transport
in liquid metals, but there is excellent agreement between the present
results and recent experimental estimates of viscosity at the melting temperature
(grey line in 3.5-3).
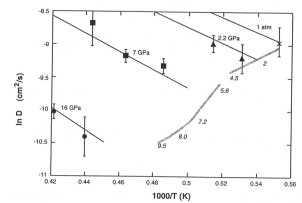 |
These results suggest that the molecular viscosity of the outer core may be as much as 105 times larger than previous estimates. The low predicted chemical diffusivity of outer core liquid metal strongly favours chemical over thermal buoyancy as the main driving force of outer core convection.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page