

The viscosities of silicate liquids at high pressures and temperatures have proven difficult to predict and to determine experimentally. Continued research into this field has been motivated by the relevance of viscosity to many deep Earth processes (both present day and past) and to furthering our understanding of silicate liquid structure. Despite what one would intuitively expect, the viscosities of silicate liquids do not simply increase with increasing pressure, but instead pass through maxima and minima. The reasons behind such behavior are poorly understood but are thought to result from changes in melt structure, ionic coordination or a change in the mechanism of viscous flow with pressure.
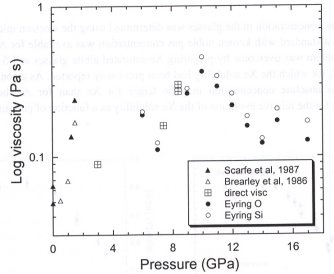
The most effective method used to determine viscosity at high pressure is that of falling sphere viscometry. However, due to small sample sizes and the inaccessibility of the sample at high pressure, direct determination of viscosity has been limited to relatively low pressures (<2.5 GPa) and to polymerised liquids with relatively high viscosities. Only through recent developments of in-situ falling sphere viscometry have measurements of more depolymerised liquids at higher pressures been possible.The use of X-ray absorbency contrast imaging with falling sphere viscometry has been successfully used over the past 2 years to measure the viscosity of jadeite (NaAlSi2O6, NBO/T = 0), sodium disilicate (Na2Si2O5, NBO/T = 1) and diopside (CaMgSi2O6,NBO/T = 2) liquids. Work at the Advanced Photon Source (ANL, Chicago, USA), led to the development of a very successful sample assembly with excellent sphere visibility and furnace stability up to 2000°C and 5 GPa for a sample volume of 4 mm3 (see 1999 Annual Report). However, the small size of the multianvil (DIA250) on that beamline has made higher pressure measurements impossible without significantly decreasing the sample volume. In the past year collaboration with scientists at the SPring8 synchrotron radiation facility (JASRI, Hyogo, Japan) has led to the further development of a new cell design that has been successfully tested to allow measurements at pressures and temperatures in the diamond stability field using the SPEED1500 multianvil press and an octahedral pressure medium with a 14 mm edge length (sample volume of approximately 10 mm3). A graphite furnace was replaced with LaCrO3resulting in the modification of the position of the MgO pressure calibrant within the assembly and the subsequent need for a second thermocouple. New data for diopside viscosity up to 8 GPa was acquired during the last allotted beam time and further determinations over 10 GPa are being pursued. An important application of the direct viscosity results is a comparison with viscosities calculated from the self-diffusion of silicon and oxygen using the Eyring equation (1998 Annual Report). The preliminary direct viscosity results for both sodium disilicate and diopside (shown in Fig. 3.5-5) liquids are consistent with those determined using the Eyring equation with silicon and oxygen self-diffusion. The consistency between the data sets validates both techniques as independent means of measuring the viscosity of silicate liquids and facilitates future work over a greater pressure and temperature range.
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page