

The heat capacity of silicate glasses and melts is of increasing importance in petrology and volcanology, as calculations involving the physical and chemical properties of magmas become more quantitative. Furthermore, heat capacity data are also important for theoretical reasons because of the close relationship between the thermodynamic and vibrational properties of condensed matter phases.
A great number of heat capacity measurements of silicate glasses and melts are published in the literature. They have been used to derive empirical equations for the calculation of the heat capacity and relative enthalpies as a function of temperature and chemical composition assuming ideal-mixing of the partial molar heat capacities of oxide components.
Calculations of the thermodynamic properties using realistic atomistic models have been reported only recently for liquids in the system NaAlSiO4 - SiO2 at very high temperatures (2500 - 4000 K) using molecular dynamics simulations.
We have performed first calculations
of the specific heat at constant volume (Cv), the specific heat
at constant pressure (Cp) and the entropy (S) below the glass
transition temperature (Tg) for several alkali silicate glasses
with composition of SiO2, Na2O·4SiO2 (NS4),
Na2O·2SiO2 (NS2) and Na2O·SiO2
(NS1) using computer models involving molecular dynamics and/or reverse
Monte Carlo simulations. The models contain from 234 to 1080 atoms. The
vibrational density of states (VDOS) of the models was calculated using
valence type harmonic potential and direct diagonalization of the dynamical
matrix. Cv was calculated using the standard statistical-mechanics
relation between Cv and VDOS, assuming completely vibrational
origin of the heat capacity. Cp was then calculated as Cp(T)
= Cv(T) + 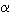 2KVT,
where the temperature dependences of the expansion coefficient
2KVT,
where the temperature dependences of the expansion coefficient 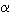 ,
the bulk modulus K and the molar volume V were taken from the literature.
,
the bulk modulus K and the molar volume V were taken from the literature.
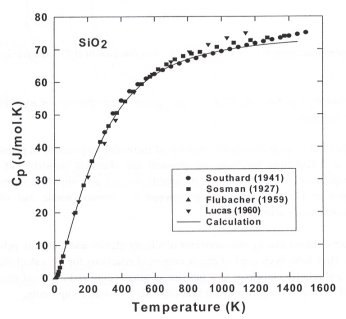
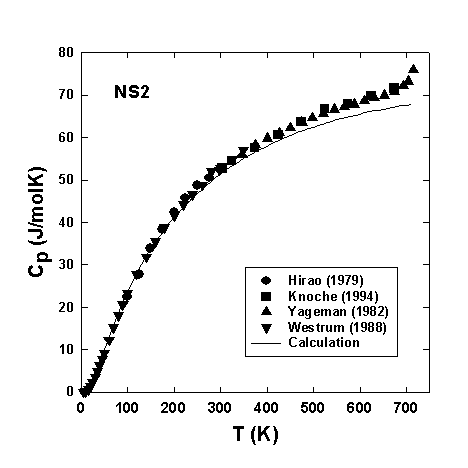
Figures 3.5-9 and 3.5-10 compare our calculated heat capacities of SiO2 and NS2 glasses,
respectively, to those experimentally measured by different authors. The
calculated Cp for amorphous SiO2 is in excellent
agreement with experiment in a wide temperature range (30 - 1200 K). For
sodium silicate glasses the calculated Cp close to Tg
remains below the Dulong-Petit limit and the discrepancy
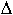 Cp
= Cpexp - Cpcal increases with
increasing degree of depolymerization.
Cp
= Cpexp - Cpcal increases with
increasing degree of depolymerization.
 |
Detailed analysis of the partial atomic heat capacities derived from the corresponding partial VDOS
shows that: (i) The bridging oxygens (BO) give the largest contribution
to Cv; with increasing degree of depolymerization the BO contribution
decreases linearly at the expense of the contributions of non-bridging
oxygens (NBO) and Na contributions. This result explains quantitatively
the experimentally observed increase of the total heat capacity with decreasing
concentration of the SiO2 component; (ii) The Na-O vibrations
contribute significantly to the heat capacity at low temperatures (T 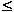 100 K) but are practically independent of T at high temperatures; (iii)
the thermodynamic properties of sodium silicate glasses are only weakly
dependent on the actual Q-species distribution and the amount of medium-range
order; (iv) the anharmonic contributions to the heat capacity at high temperatures
increase with increasing degree of depolymerization.
100 K) but are practically independent of T at high temperatures; (iii)
the thermodynamic properties of sodium silicate glasses are only weakly
dependent on the actual Q-species distribution and the amount of medium-range
order; (iv) the anharmonic contributions to the heat capacity at high temperatures
increase with increasing degree of depolymerization.
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page