

A detailed knowledge of the rates and mechanisms of mineral dissolution is essential to our understanding of many magmatic processes including assimilation, magma mixing and xenolith transport and alteration. Results presented in the 1999 Annual Report showed that the geometry of the mineral - melt system can have a strong effect on dissolution rates. In addition, further analysis of these experiments indicates that, in very silica undersaturated melts, quartz dissolution is controlled by interface kinetics rather than by diffusion in the melt. This is the opposite of the predictions made by current mineral dissolution models.
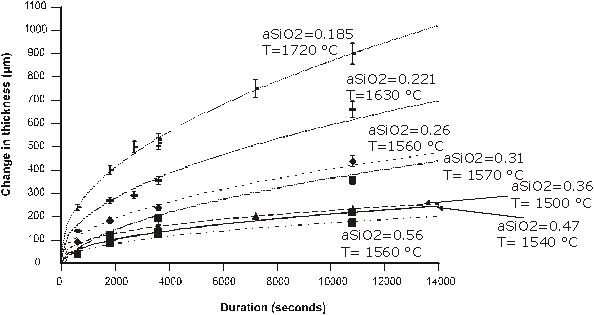
In order to investigate the effects of melt compositon on mineral dissolution rates, quartz has been dissolved in melts in the CMAS (CaO - MgO - Al2O3 - SiO2) system at 1.5 GPa with 20°C superheat. The melts range from olivine normative to quartz normative and have silica activities from 0.18 to 0.56. The dissolution rate data (Figure 3.6-3) show that dissolution rates decrease with increasing silica activity and are approximately proportional to the square root of time suggesting that dissolution is controlled by diffusion in the melt.
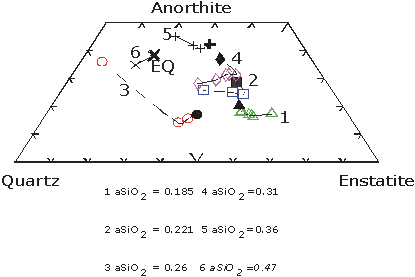
Analysis of the interface melts for all the experiments shows that in all cases where the silica activity of the solvent is 0.36 or less, the rate controlling mechanism in not diffusion since the interface melt never reaches saturation, defined by long duration quartz + solvent experiments (Figure 3.6-4). In addition, modelling of the compositional profiles suggests that diffusion rates decrease as a function of time.
In contrast to melts with silica activites below 0.36 silica-rich melts show interface saturation after approximately 30 minutes (Figure 3.6-4) after which dissolution is controlled by diffusion in the melt. These results suggest that the current models of dissolution, in which the rate controlling mechanism is always assumed to be diffusion of components away from the crystal - melt interface, are incorrect and that the degree of undersaturation of the solvent melt in the dissolving component is extremely important.
 |
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page