

The electrical conductivities of olivine and its high-pressure polymorph wadsleyite differ by about two orders of magnitude. Using complex impedance spectroscopy, we are able to measure electrical conductivity as the phase transformation from olivine to wadsleyite occurs in real time at high-pressure and high-temperature conditions in a multi-anvil apparatus. The bulk electrical conductivity of a partially transformed sample does not necessarily scale in proportion to the volume fractions of the two phases. How the two phases are distributed also influences the bulk conductivity. To address this, we have also carried out quench experiments in order to analyze the microstructural development at various stages during the transformation. In particular, we aim to recognize the electrical signature in the in-situ measurement that corresponds to the formation of an interconnected network of the more conductive wadsleyite phase along grain boundaries. At this point, not only should the bulk electrical properties of the two-phase sample change, but other properties as well, such as the overall strength of the aggregate material. Additionally, with a proper understanding of the microstructural development during transformation, the in-situ impedance measurement then promises to be an effective means of obtaining kinetic information for this phase transformation and others, which also exhibit measurable changes in electrical conductivity.
High-pressure experiments are being carried out in a 1000-tonne multi-anvil apparatus using 14 mm (edge length) MgO octahedra with 8 mm TEL tungsten carbide cubic anvils (32 mm). Previously hot-pressed San Carlos olivine starting materials are pressurized to 8 GPa and re-hot-pressed at 1200°C for 2.5 hrs. Temperature is then reduced to 600°C before increasing the pressure to 15 GPa. At 15 GPa, T is increased rapidly (300°C/min) to the desired temperature for observation of the transformation. Complex impedance spectra are collected over a relatively small range of frequencies (500 kHz to 100 Hz, 4 points per decade in frequency) in order to achieve a high sampling rate (about 1 spectrum every 45 seconds). This frequency range encompasses a majority of the impedance spectra of both olivine and wadsleyite.
Impedance data collected over a period of several hours show the progression from the low conductivity phase olivine to the more conductive phase wadsleyite. The impedance spectra can be approximated well by a simple parallel RC equivalent circuit in order to determine the bulk electrical conductivity of the sample at any point in time during the transformation.
Because each individual spectrum is ideally semi-circular, the value of the real impedance (Z') at the lowest frequency, where the imaginary impedance (Z'') lies furthest along the x-axis and closest to zero, provides a good approximation of the bulk resistance. Analytical fits to several selected spectra have shown that the lowest frequency Z' value was within error of the best fit value for the resistance. We are therefore able to use the data at this frequency alone to monitor the change in bulk conductivity as a function of time (this is potentially important for improving time resolution in cases of faster kinetics, i.e. higher temperatures).
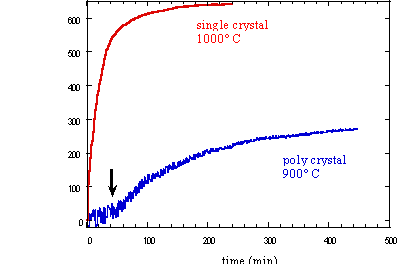
Our sample has an initial (t = 0) bulk conductivity higher than that expected for olivine. Because, at zero time, we are actually measuring outside of the stability field of olivine, its enhanced bulk conductivity probably is an effect of the high pressures. The final bulk conductivity is at any rate consistent with that previously measured for wadsleyite. The effect of temperature and texture on the conductivity is illustrated in Figure 3.7-5. The single crystal experiment at 1000°C shows a rapid increase in conductivity at the very onset of the transformation. The conductivity already reaches a plateau after about 200 minutes, indicating complete transformation. Conductivity changes more slowly with time in the 900°C experiment with polycrystalline olivine; after 400 minutes it still has not reached a plateau value. A unique feature in the measurement of the polycrystalline sample can be seen after about 50 minutes (arrow in Fig. 3.7-5) where the rate of increase in conductivity abruptly changes. We attribute this feature as the point in the transformation when an interconnected network of wadsleyite has been formed along grain boundaries. No such feature can be observed in the trace of the single crystal experiment in which only homogeneous intracrystalline nucleation and growth mechanisms are expected.
Analyses of partially transformed samples from our parallel quench study help us to understand how the amounts and distribution of the two phases relates to the electrical response of the bulk sample. Powder X-ray diffraction enables us to estimate the extent of the transformation in terms of the volume fractions of the two phases present. We have also employed micro-Raman spectroscopy as a way of mapping the distribution of the phases since they are virtually indistinguishable chemically. Results from these analyses indeed show that interconnectivity occurs at very early stages of transformation that correspond to low volume fractions of wadsleyite, and support our conclusion that it is possible to extract some ofthe details of microstructural development of phase transformations using this in-situ technique. Continued efforts to extract more quantitative information, particularly related to transformation kinetics, will prove useful towards our understanding of subduction processes.
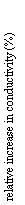  |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page