

The alkali/aluminum ratio of granitic melts is one of the most important
compositional parameters controlling their physico-chemical behaviour in
petrogenetic processes. The physical properties of granitic melts are strongly
influenced by this ratio, especially the viscosity. At alkali/aluminum
ratios greater than 1 the viscosity drops very strongly. At alkali/aluminum
ratios less than 1 the picture is less clear but no sharp drop is observed.
The incorporation of water in granitic melts with alkali/aluminum ratios
near 1 also causes a strong decrease in viscosity. The question remains
however as to how the viscosity of granitic melts with varying alkali/aluminum
ratios (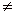 1) are influenced by water addition.
1) are influenced by water addition.
Due to the development of dilatometric methods for the investigation
of the viscosity of hydrous granitic melts at 1 atm, this question can
now be experimentally addressed in the viscosity range where the largest
effects are expected. Figure 3.6-2 illustrates data for the viscosities
of various melts with added water.
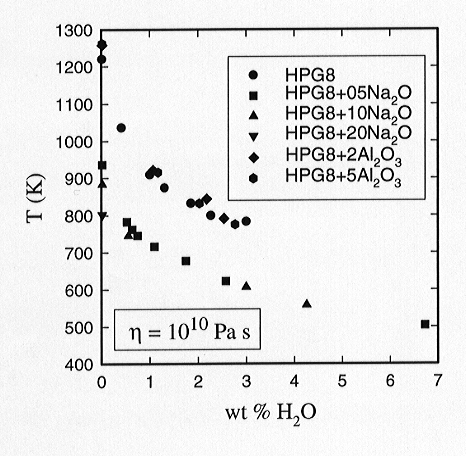 |
Fig 3.6-2: The influence of water on the viscosity of haplogranitic melts at 1 atm pressure for a constant viscosity. The influence of water is similar for the metaluminous and peraluminous melts. In contrast, the effect of water depends significantly on the alkali/aluminum ratio for the peralkaline melts, especially at low water contents. |
Five base compositions are presented representing the addition or subtraction of varying amounts of Al2O3 to a base metaluminous base composition. A strong decrease of the viscosity of the melt is observed for all compositions investigated. The effect of water on the viscosities of the two peraluminous melts appears to be well described by the case for the addition of water to the metaluminous base melt. The viscosities of slightly peraluminous and metaluminous melts with equivalent water contents are similar. Thus the recent viscosity model of Hess and Dingwell can probably be used with confidence for peraluminous systems. For the peralkaline melts the situation is quite different. The decrease in viscosity with addition of water to a melt with excess Na2O is less strong than that observed for the addition of water to the metaluminous base composition. Furthermore, the viscosities of increasingly peralkaline melts appear to be progressively less sensitive to the addition of water, such that a doubling of the excess alkali content at high peralkalinity produces no further significant decrease in melt viscosity.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page