
Synthesis of paracrystalline diamond
H. Tang, X. Yuan, Y. Cheng, H. Fei, F. Liu, T. Liang, Z. Zeng, T. Ishii, M.-S. Wang, T. Katsura, H. Sheng, H. Gou
Nature 599, 605–610 (2021). https://doi.org/10.1038/s41586-021-04122-w
Diamond is a high-pressure crystalline form of carbon. Each carbon atom is bonded by four covalent sp3 bonds, which is a distinctive difference from low-pressure carbon forms, whose carbon atoms have three covalent sp2 bonds. The strong sp3 bonds are the origin of the high hardness of diamond. Because of the long-range crystal structure, the physical properties of diamond are anisotropic. Therefore, it is desirable to synthesize a non-crystalline, fully sp3-bonded form of carbon. We successfully synthesized non-crystalline fully sp3-bonded carbon using advanced high-pressure technology. Detailed structural analysis clarified that this material is neither crystalline nor amorphous, but paracrystalline, having no long-range but medium-range order. Thus, this material is a novel form of carbon and is referred to as ‘paracrystalline diamond’.
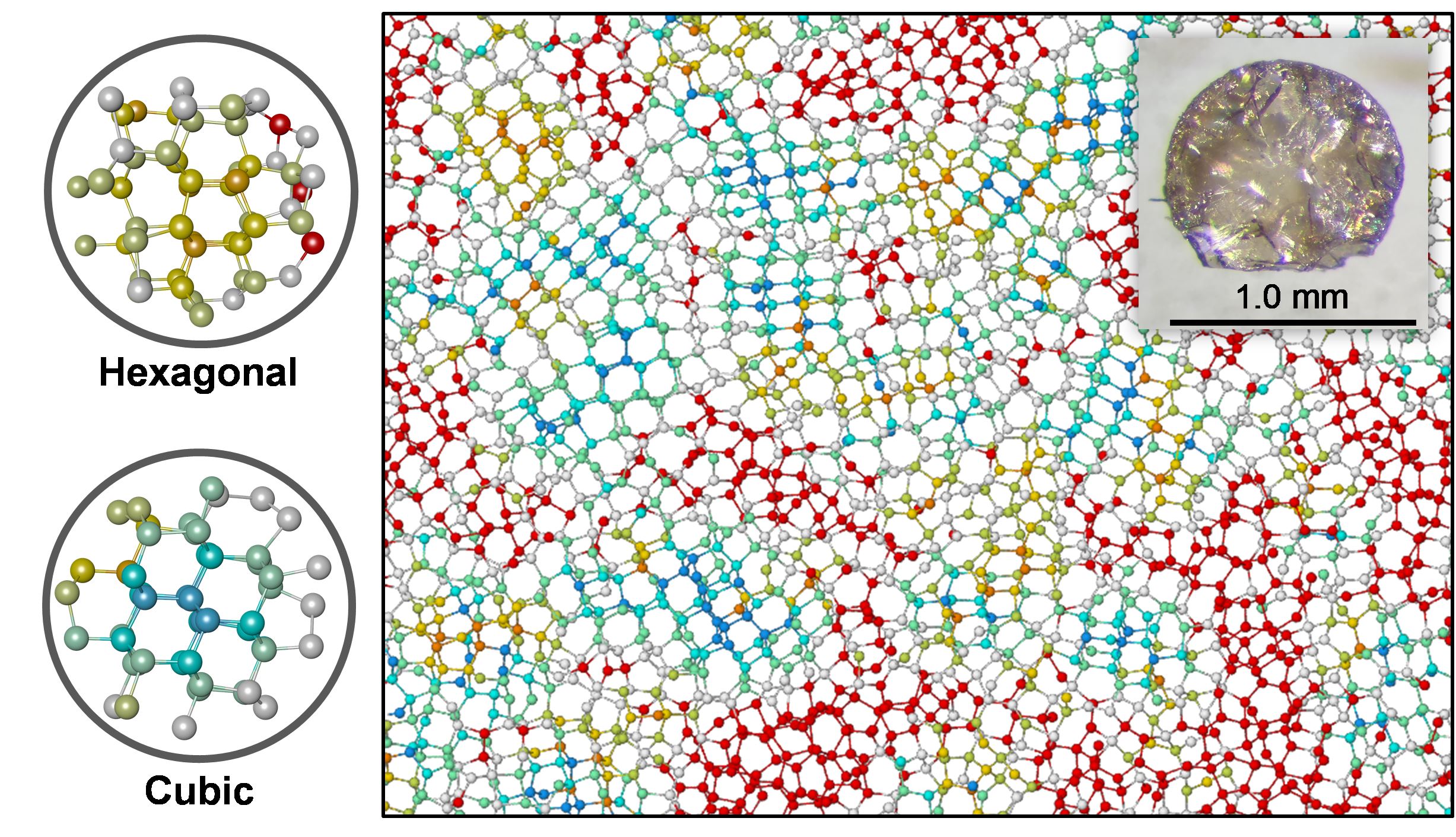
Figure: Structural model of paracrystalline diamond from MD simulation. Colors represent different types of atomic packing. The turquoise, gold, and red atoms represent cubic-, hexagonal-like, and disordered atomic packing within two atomic shells, respectively.
Inset: Optical microphotograph of ‘paracrystalline diamond’. Its appearance is indistinguishable from crystalline diamond.
Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de