

The suggestion that significant amounts of water exist in phases within the transition zone has generated a large number of studies to investigate the stability and properties of hydrous phases at high pressure. Many of these hydrous phases contain iron, yet few studies have addressed the question of its oxidation state. To address this problem, we used Mössbauer spectroscopy to characterize the oxidation state and crystal chemistry of iron in some hydrous phases relevant for the transition zone.
Single crystals of hydrous majorite and two forms of wadsleyite were
synthesised using a multianvil press from starting mixtures of Mg-free
KLB-1 peridotite gel, brucite and FeO. For comparison, "dry" samples of
polycrystalline majorite and wadsleyite were synthesised in the system
FeO-MgO-SiO2 from either orthopyroxene or olivine synthetic
starting materials at conditions of high oxygen fugacity. Fe3+/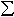 Fe
was determined using Mössbauer spectroscopy, where the milliprobe
technique was used to measure values for the single crystals.
Fe
was determined using Mössbauer spectroscopy, where the milliprobe
technique was used to measure values for the single crystals.
Values of Fe3+/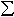 Fe for the "dry" phases are relatively low
(5-20%), while Fe3+/
Fe for the "dry" phases are relatively low
(5-20%), while Fe3+/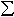 Fe values for the hydrous phases are quite
high (50-100%). The high Fe3+/
Fe values for the hydrous phases are quite
high (50-100%). The high Fe3+/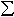 Fe values are also consistent
with the results of single crystal refinements using X-ray diffraction.
The most likely explanation for the high Fe3+/
Fe values are also consistent
with the results of single crystal refinements using X-ray diffraction.
The most likely explanation for the high Fe3+/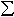 Fe values in
the hydrous phases is the presence of H in the crystal structure, although
it is possible that additional components such as Ca, Na, Al, Ti and Cr
in the hydrous phases could also have an effect. If these phases occurred
within the transition zone, Fe may occur predominantly as Fe3+.
This could have significant effects on physical and chemical properties,
for example elasticity, transport properties, mechanical behaviour, and
trace-element partitioning and should be considered in constructing mineralogical
models for the transition zone from geophysical data.
Fe values in
the hydrous phases is the presence of H in the crystal structure, although
it is possible that additional components such as Ca, Na, Al, Ti and Cr
in the hydrous phases could also have an effect. If these phases occurred
within the transition zone, Fe may occur predominantly as Fe3+.
This could have significant effects on physical and chemical properties,
for example elasticity, transport properties, mechanical behaviour, and
trace-element partitioning and should be considered in constructing mineralogical
models for the transition zone from geophysical data.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page