

Titanium dioxide (TiO2) exists in nature as the minerals rutile, anatase and brookite. Rutile is an important rock-forming mineral and the most abundant TiO2 polymorph in nature. Relative to rutile there are fewer investigations of the anatase phase, although it constitutes most of the commercially produced material (e.g. as white pigment for paints, plastics and paper).
The present work is an experimental and theoretical study of the high-pressure behavior of TiO2, using anatase as the starting material. Precise unit-cell parameters of anatase have been determined by single-crystal X-ray diffraction. However, due to a phase transition, the crystal breaks and cannot be investigated further. Using powder X-ray diffraction, it has been possible to study the high-pressure behavior of both anatase and its high-pressure polymorphs, albeit with a lower resolution. Due to the scatter of published data for the equation-of-state parameter of anatase, we have also investigated this polymorph theoretically using a quantum-mechanical method.
The results are highly consistent, giving zero-pressure bulk moduli Bo between 179 and 190 GPa. Single-crystal anatase transforms to the 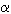 -PbO2 structure at about 4.5 GPa. The zero-pressure bulk modulus and its pressure derivative were determined by a fully weighted least-squares fit of the Birch-Murnaghan equation of state to the PV data set. This gives Bo = 179(2) GPa and Bo' = 4.5(1.0) with Vo = 136.277(5) Å3. The transformation to the
-PbO2 structure at about 4.5 GPa. The zero-pressure bulk modulus and its pressure derivative were determined by a fully weighted least-squares fit of the Birch-Murnaghan equation of state to the PV data set. This gives Bo = 179(2) GPa and Bo' = 4.5(1.0) with Vo = 136.277(5) Å3. The transformation to the 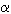 -PbO2 phase is suppressed in the polycrystalline material at room temperature, probably due to the presence of crystal defects. Polycrystalline anatase transforms to the baddeleyite type structure at about 13 GPa. Upon decompression, the baddeleyite-type phase transforms to the
-PbO2 phase is suppressed in the polycrystalline material at room temperature, probably due to the presence of crystal defects. Polycrystalline anatase transforms to the baddeleyite type structure at about 13 GPa. Upon decompression, the baddeleyite-type phase transforms to the 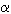 -PbO2 phase at about 7 GPa. The zero-pressure bulk moduli are 258(10) GPa for the
-PbO2 phase at about 7 GPa. The zero-pressure bulk moduli are 258(10) GPa for the 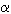 -PbO2 phase and 290(15) GPa for the baddeleyite phase.
-PbO2 phase and 290(15) GPa for the baddeleyite phase.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page