

Silicon nitride (Si3N4) is one of the most common non-oxide ceramic materials. The outstanding combination of hardness, strength and chemical inertness has led to considerable commercial success in widespread applications such as cutting tools, bearings, turbines and turbo-charger rotors. In addition, Si3N4 thin films are now commonly used for electrical insulation and heat transport in electronic components. Both 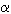 -Si3N4 and
-Si3N4 and 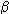 -Si3N4 are hexagonal and have been produced synthetically for many years. In 1995, they were discovered to occur naturally in some meteorites. These polymorphs are constituted by different arrangements of SiN4-tetrahedra, three of which share a common N atom. Recently, we succeeded in synthesizing a third polymorph of silicon nitride, with cubic symmetry, in a diamond anvil cell from elemental silicon and nitrogen. Using large volume multianvil apparatus, we have now been able to make c-Si3N4 starting from amorphous precursors at conditions of 15 GPa and 1800°C. The recovered samples are white, with a fine-grained, glassy appearance. Analyses by Raman, FTIR and XRD show that spinel type silicon nitride is the major constituting phase of all samples subjected to a pressure of 15 GPa. One experiment, conducted at 10 GPa, resulted in the recovery of solely
-Si3N4 are hexagonal and have been produced synthetically for many years. In 1995, they were discovered to occur naturally in some meteorites. These polymorphs are constituted by different arrangements of SiN4-tetrahedra, three of which share a common N atom. Recently, we succeeded in synthesizing a third polymorph of silicon nitride, with cubic symmetry, in a diamond anvil cell from elemental silicon and nitrogen. Using large volume multianvil apparatus, we have now been able to make c-Si3N4 starting from amorphous precursors at conditions of 15 GPa and 1800°C. The recovered samples are white, with a fine-grained, glassy appearance. Analyses by Raman, FTIR and XRD show that spinel type silicon nitride is the major constituting phase of all samples subjected to a pressure of 15 GPa. One experiment, conducted at 10 GPa, resulted in the recovery of solely 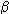 -Si3N4 providing a constraint on the location of the phase boundary.
-Si3N4 providing a constraint on the location of the phase boundary.
SEM and TEM investigations revealed a homogeneous crystalline material including minor amounts of an amorphous phase. The c-Si3N4 crystallites are either of (distorted) octahedral shape or platelets with hexagon base planes, exhibiting aspect ratios close to 1. EDX analysis revealed no elements other than Si, N and O. Because no crystalline phases other than the spinel were detected by XRD or spectroscopic analysis, we suspect that any contamination of the samples is in the form of an amorphous SiO2 or SiNxOy phase. The material is very likely identical to the amorphous 10 to 50 nm thick layers surrounding c-Si3N4 grains, which can be seen in TEM bright field images. Quantitative electron microprobe analyses are in good agreement with bulk chemical analysis. A mapping of nitrogen and silicon indicates a homogeneous distribution of these elements over the whole sample cross section. In contrast, oxygen is inhomogenously distributed throughout the samples, with greater concentrations located near the sample/capsule interface. This most likely indicates that diffusion of oxygen from the surrounding oxide materials through the capsule material into the precursor material is occurring during the heating phase of the experiments.
An X-ray powder diffractogram of the cubic phase was obtained in transmission geometry with CoK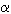 1-radiation. The full-width half maximum of the c-Si3N4 lines are considerably narrower than those of the Si-standard. Rietveld refinement results in site occupancies which deviate from theoretical Si3N4 in the spinel structure, which we attribute to the presence of oxygen in the crystalline lattice. Sealing the capsules under an inert atmosphere and the facility to implement oxygen "getters" around the sample during synthesis is expected to reduce oxygen contamination in future synthesis experiments. As cubic Si3N4 is expected to have novel material properties, as do other cubic nitride phases (e.g. c-BN,
1-radiation. The full-width half maximum of the c-Si3N4 lines are considerably narrower than those of the Si-standard. Rietveld refinement results in site occupancies which deviate from theoretical Si3N4 in the spinel structure, which we attribute to the presence of oxygen in the crystalline lattice. Sealing the capsules under an inert atmosphere and the facility to implement oxygen "getters" around the sample during synthesis is expected to reduce oxygen contamination in future synthesis experiments. As cubic Si3N4 is expected to have novel material properties, as do other cubic nitride phases (e.g. c-BN, 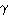 -Ge3N4), the large volume synthesis method is not only important for controlling the sample's chemical environment, but also for generating large enough samples for various methods of property testing.
-Ge3N4), the large volume synthesis method is not only important for controlling the sample's chemical environment, but also for generating large enough samples for various methods of property testing.
We also plan to investigate the phase relations of the solid solution series (Si,Ge)3N4, in which we expect lower pressures are required to form spinels due to the larger Ge4+ cation occupying the octahedral site. Other plans include examination of the system Si-C-N, as precursors with stoichiomtetries SiC2N4 and Si2CN4 are already available. In particular, the silicon(carbodiimide)nitride Si2CN4 provides an appealing Si:C ratio, where all Si atoms would occupy octahedral sites, while all carbon atoms would remain tetrahedrally co-ordinated, if squeezed into the spinel structure. According to theoretical considerations of Cohen, both the coordination number and the strength of the C-N bond will contribute to the hardness of a covalent solid like c-Si2CN4.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page