

Chemical partitioning between silicate and carbonatite melts has been investigated using a centrifuge furnace and autoclave. Results for major and trace elements have been reported in previous Annual Reports. Here, the first oxygen isotope partitioning experiments between silicate and carbonatite melts using the centrifuge method are presented.
The silicate fraction was split into several aliquots for several δ18O measurements. Oxygen was extracted by fluorination following the methods of Clayton and Mayeda (1963):
(1) Approximately 10 mg-samples were loaded in nickel tubes placed in a dry box; (2) the nickel tubes were carefully evacuated and degassed at 150°C for 3 hours; (3) then BrF5 was added and the temperature of the nickel tubes set to 550°C for 8 hours; (4) the released oxygen was purified with a molecular sieve at -196°C and the oxygen yield was precisely measured with a manometer.
The carbonate phase was separated in large fragments to minimize water adsorption on surfaces. One fragment was carefully weighed and loaded into a degassed platinum tube. The Pt tube was then loaded into a degassed quartz tube. The quartz tube was immediately connected to a vacuum line and evacuated to a vacuum better than 10-5 mbar for more than 4 hours (> 4). The complete decarbonation was obtained in less than about 45 minutes by heating the quartz tube at 1000°C and trapping the released CO2 in a trap at -196°C. Then the quartz tube was sealed under vacuum to preserve the residual oxide from humidity. The trapped CO2 was liberated at -135°C and the CO2 yield measured manometrically. The released water was liberated at room temperature and reduced into H2 with hot uranium. The H2 yield was measured manometrically.
The sealed quartz sealed tube was placed in a dry box and broken for loading the platinum tube (containing the residual oxide) into a nickel tube for fluorination (to extract the oxygen of the residual oxide). Any quartz fragments possibly produced were carefully extracted with a fine brush. The Pt container was then transferred into the nickel tube. The oxygen extraction was performed as for the silicate phases.
All the isotopic analyses were made on an Optima VG mass spectrometer. The results are reported with the traditional δ notation (per mil unit). Reproducibilities are routinely better than 0.1‰ for both CO2 (from decarbonation) and O2. The isotopic fractionation between the carbonate and silicate phases has been calculated and is expressed as Δ18O c-s. At 915°C, two values are obtained: 1.56±0.90‰ and 1.97± 0.20‰. At 965°C only one value, 1.51±0.15‰, is acceptable and at 1015°C we calculate 0.99± 0.63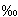 . The Δ18O c-s is in agreement with the Δ18O calcite-melilite determination of Conway and Taylor (1969) who found 2-3
. The Δ18O c-s is in agreement with the Δ18O calcite-melilite determination of Conway and Taylor (1969) who found 2-3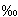 between 800 and 600°C. Our calculated Δ18O c-s decreases with increasing temperature as predicted by isotope fractionation theory. Further experiments will concentrate on chemical compositional dependence of the fractionation factor.
between 800 and 600°C. Our calculated Δ18O c-s decreases with increasing temperature as predicted by isotope fractionation theory. Further experiments will concentrate on chemical compositional dependence of the fractionation factor.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page