

GeO2-glass is often used as a SiO2 analogue as it forms a fully polymerized tetrahedral network, but undergoes structural changes at much lower P-T conditions than SiO2. For example, the glass transition temperature (Tg) for GeO2-glass is 580°C whereas Tg for SiO2 is approximately at 1200°C, a temperature where experimental access is difficult.
Although this glass is nominally dry, it is important to determine the exact amount of water, because a few tenths of weight percent water can dramatically influence such rheological properties as viscosity. To determine the H2O content we could not use Karl-Fischer-titration, as this method is restricted to > 0.2 wt% H2O and IR-spectroscopy was excluded because no information about the molar extinction coefficient of H2O in GeO2-glass could be found in the literature. Therefore we determined the amount of water in our samples by the 15N method. In this method the sample is exposed to a 15N beam which reacts with 1H as in the following nuclear reaction:
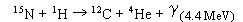
This 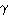 -radiation is characteristic for the presence of hydrogen, therefore the concentration of hydrogen can be measured by counting the
-radiation is characteristic for the presence of hydrogen, therefore the concentration of hydrogen can be measured by counting the 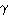 -ray yield.
-ray yield.
Seven disks of the glass were prepared for measurement in the following fashion. We synthesized the GeO2-glass in a Pt crucible from pure GeO2 powder (99.99%). Cylinders were drilled out from the glass block and cut into disks of 3 mm height and 8 mm diameter. The samples were polished with parallel faces and stored in a desiccator. Four samples were treated with HF to ensure a water-free surface. Figure 3.4-12 shows the water content versus the depth for the untreated and the HF-treated samples. As one can see the untreated samples reach the water bulk composition (70 ppm) at approximately 2000 nm. For the treated samples this level is reached in less than 200 nm. This effect has to be taken into account when an investigation of a sample involves the surface and the near surface region.
Infrared spectra were measured in the near IR range between 3000 - 4000 cm-1. All samples showed a single asymmetric absorption peak at 3560 cm-1 due to the fundamental OH-stretching vibration. The density was determined by a Mettler Toledo density balance. From these data, one can calculate the molar extinction coefficient using the Beer-Lambert law,
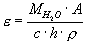
where MH2O is the molecular weight of water, A the peak height of the absorbance band, c the water content in wt%, h the thickness of the sample and 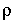 the density. From this the linear molar extinction coefficient for H2O in GeO2 glass was determined to be 132 l/mol cm. The 15N method is a powerful tool to determine small amounts of water in solids, especially when other methods like Karl-Fischer-titration or IR spectroscopy cannot be used.
the density. From this the linear molar extinction coefficient for H2O in GeO2 glass was determined to be 132 l/mol cm. The 15N method is a powerful tool to determine small amounts of water in solids, especially when other methods like Karl-Fischer-titration or IR spectroscopy cannot be used.
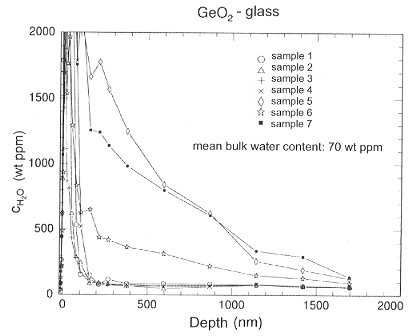 |
Fig. 3.4-12: Water concentration profile of GeO2-glass. The HF-treated samples (1-4) reach the water bulk composition within the first 200 nm. For the untreated samples (5-7) this depth is approximately 2 µm. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page