

A number of studies at the Bayerisches Geoinstitut have provided exciting results on the pressure dependence of hydroxyl solubility in "nominally anhydrous minerals" (NAMs). Although not as abundant in the Earth as olivine and pyroxene, rutile (TiO2) is the "wettest" NAM yet found in nature, with hydroxyl contents ranging up to 0.8 wt% H2O. The goal of the present study is to measure the solubility of hydroxyl in rutile as a function of pressure, temperature, and defect chemistry. Rutile can incorporate a large number of substitutional trivalent (Cr, Fe,V, Al) and pentavalent (Nb, Ta) cations, making it an ideal candidate for such study. Reconnaissance multianvil experiments have been conducted using pure TiO2 powder (+H2O) as the starting material. The samples were held at 1200°C and 6, 8, or 10 GPa for 24 hours. Talc and Ni/NiO powders were placed in an outer capsule in order to influence H2O and O2 fugacities, respectively. All of the experiments yielded coarse (ca. 300 µm average grain size) aggregates of TiO2 exhibiting an intense blue color in plane polarized light; the color is probably due to charge transfer between Ti3+ and Ti4+ ions. At 6 and 8 GPa rutile was the product phase, whereas at 10 GPa the dominant product phase was 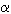 -PbO2-structured "TiO2-II", the high-pressure polymorph of rutile. FTIR measurements on the latter material failed to detect the presence of hydrogen. Unpolarised FTIR measurements on the samples synthesized at 6 and 8 GPa, however, show two sharp peaks at 3286 and 3325 cm-1, associated with hydrogen defects (Fig. 3.4-13). These peaks are superimposed on a much larger, broad band with a maximum between 6000-7000 cm-1, caused by intervalence charge transfer between Ti3+ and Ti4+. The concentration of hydroxyl in both samples is ˜0.3 wt% (calculated using Beer's law, the known extinction coefficient for rutile, and using an orientation factor of 1/3 to account for unpolarised radiation). Because hydrogen bonding in rutile is strongly polarized, this measurement probably represents a minimum value for the concentration. The high solubility of hydroxyl in rutile even in the pure system is surprising, as previous work has shown hydroxyl contents to be strongly correlated with minor element concentrations. However, the presence of Ti3+ ions in reduced TiO2 may facilitate the incorporation of OH- groups. Future solubility experiments will be conducted using well-characterized, oriented, synthetic single crystals with three different compositions: nominally pure, doped with 0.02 wt% Al2O3, and doped with 0.1 wt% Fe2O3.
-PbO2-structured "TiO2-II", the high-pressure polymorph of rutile. FTIR measurements on the latter material failed to detect the presence of hydrogen. Unpolarised FTIR measurements on the samples synthesized at 6 and 8 GPa, however, show two sharp peaks at 3286 and 3325 cm-1, associated with hydrogen defects (Fig. 3.4-13). These peaks are superimposed on a much larger, broad band with a maximum between 6000-7000 cm-1, caused by intervalence charge transfer between Ti3+ and Ti4+. The concentration of hydroxyl in both samples is ˜0.3 wt% (calculated using Beer's law, the known extinction coefficient for rutile, and using an orientation factor of 1/3 to account for unpolarised radiation). Because hydrogen bonding in rutile is strongly polarized, this measurement probably represents a minimum value for the concentration. The high solubility of hydroxyl in rutile even in the pure system is surprising, as previous work has shown hydroxyl contents to be strongly correlated with minor element concentrations. However, the presence of Ti3+ ions in reduced TiO2 may facilitate the incorporation of OH- groups. Future solubility experiments will be conducted using well-characterized, oriented, synthetic single crystals with three different compositions: nominally pure, doped with 0.02 wt% Al2O3, and doped with 0.1 wt% Fe2O3.
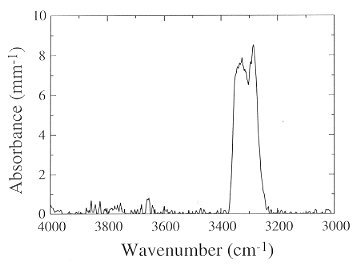 |
Fig. 3.4-13: Baseline-corrected, unpolarised FTIR spectrum of rutile synthesized at 6 GPa. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page