

Phase transformations of enstatite, the second most abundant phase in subducting lithosphere, must be considered when investigating the reactions associated with subduction and their effects on physical properties. In order to investigate the mechanisms and the transformational behaviour of enstatite and forsterite, multianvil experiments were performed on a model harzburgite (60 vol% forsterite, 40 vol% enstatite) at temperatures and pressures in the range 1000 - 1650 °C and 16 - 21 GPa.
Kinetic investigations of the reactivity of hot-pressed enstatite-forsterite
mixtures show a strongly pressure-dependent metastability of enstatite.
Whereas forsterite reacts rapidly to ß-phase
at 1200 °C and 17 GPa, enstatite survives metastably up to 20 GPa and
1550 °C. The sluggishness of the disproportionation reaction of enstatite
to ß-phase + stishovite is probably due to the
difficulty in nucleating stishovite. In experiments at 21 GPa and 1250
°C for 3.5 to 15 hours, enstatite reacted completely to MgSiO3-ilmenite
plus a symplectite of ß-phase plus stishovite,
whereas in an experiment of one hour duration, enstatite partially transformed
to ß-phase plus stishovite without MgSiO3-ilmenite.
To investigate the mechanisms of the various enstatite breakdown reactions,
experiments were run at lower temperatures (1150 °C and 21 GPa) to
preserve some metastable enstatite in contact with the product phases.
In a sample reacted for 70 minutes at these P-T conditions, enstatite was
detected by X-ray diffraction along with all of the product phases (ilmenite,
stishovite, γ-phase and ß-phase).
TEM-investigations of this sample (Fig. 3.1-2) show enstatite in contact
with ilmenite and γ-phase + stishovite symplectite.
The direction of reaction can be inferred from the convexity of the interphase
boundaries. In this example (Fig. 3.1-2) convex boundaries between enstatite
and ilmenite and ilmenite and γ-phase indicate
that enstatite transformed polymorphically to ilmenite which subsequently
reacted to γ-phase + stishovite. The boundary
between enstatite and γ-phase + stishovite symplectite
indicates a direct disproportionation reaction of enstatite to symplectite.
These microstructures demonstrate the existence of a branched reaction
path where both reactions occur simultaneously. The fact that ilmenite
breaks down to γ-phase + stishovite under these
conditions as well as at 1250 °C and 21 GPa indicates that it was either
metastable when it formed or it became metastable during transformation.
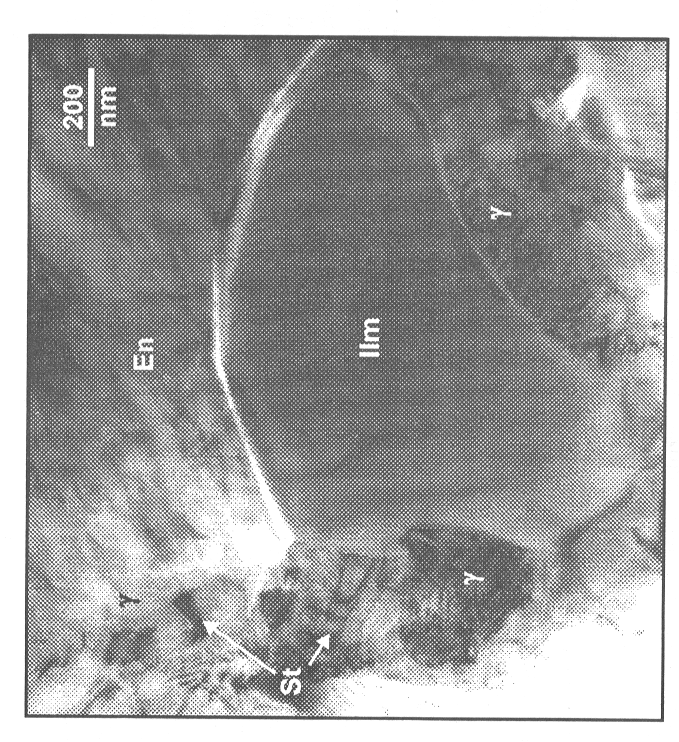 |
Fig. 3.1-2: TEM micrograph showing ilmenite (llm) in contact with enstatite (En) as well as the contacts of a developing γ-Mg2SiO4 + stishovite (St) symplecitite with the enstatite and ilmenite, respectively. The convex grain boundaries between ilmenite and the symplectite towards the enstatite demonstrate the simultaneous reaction of enstatite on two different reaction paths. Convex grain boundaries from the symplectite towards the ilmenite indicate breakdown of ilmenite to γ-phase + stishovite. |
One possible explanation for the metastability of the ilmenite phase is a localized pressure drop during the experiment caused by the large volume decrease (~11 %) of the enstatite to ilmenite transformation. Such a pressure drop is possible if plastic deformation of the sample is too slow to accommodate the decreasing volume around transforming grains. Such phenomena have been reported from in-situ multianvil X-ray diffraction studies. Depending on the relative rates of transformation and deformation, run conditions could change from the stability field of ilmenite into the stability field of γ-phase + stishovite, causing both ilmenite and enstatite to transform to the γ-phase + stishovite symplectites. One problem with this explanation is that the disproportionation breakdown of enstatite has been shown to be very sluggish due to the difficulty of nucleating stishovite at temperatures less than 1550 °C. The presence of ilmenite may decrease the activation energy barrier for stishovite nucleation, thereby allowing the direct transformation of enstatite to γ-phase + stishovite to occur. An additional problem with this scenario is that it does not explain the direct reaction of enstatite to ß-phase + stishovite at 1250 °C and 21 GPa.
An alternative explanation is that the ilmenite formed metastably as an intermediate phase in the reaction of enstatite to γ-phase + stishovite. This implies that most of the phase diagrams for the MgSiO3 system are wrong and that the stability field of γ-phase + stishovite extends to 21 GPa, which is consistent with the phase equilibria data of Yusa et al. (1993, JGA). In this explanation, the ilmenite phase forms metastably because of a significantly lower activation energy for the polymorphic reaction compared with the disproportionation reaction to γ-phase + stishovite. The reaction sequence can be considered as an Ostwald-step process where the free energy of the system is lowered in steps as a result of lower activation energies for the two-step process. The intermediate ilmenite apparently lowers the activation energy for stishovite nucleation, allowing the direct transformation to γ-phase + stishovite to occur simultaneously with the two-step reaction. The importance of nucleation barriers in these high-pressure reactions is supported by the numerous orientation relations and coherent nucleation mechanisms seen in high pressure transformations (Annual Reports 1993 and 1994). The transformation of enstatite to ß-phase + stishovite at 1250 °C and 21 GPa can also be explained by an Ostwald-step process if the ß-phase occurs as a metastable intermediate phase relative to the stable γ-phase.
To distinguish between these two possible explanations, further phase equilibria experiments are required to determine if the stability field of γ-phase + stishovite extends to 21 GPa. Additional kinetic experiments and TEM investigations are also required to determine how the two step breakdown reaction assists in the nucleation of stishovite.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page