

Magnesiowüstite is one of the most important candidate minerals in the lower mantle. Therefore, the partitioning of elements between magnesiowüstite, silicate melt, and molten iron is important for understanding the fractionation by melting in the deep mantle during core formation and during a subsequent high-temperature stage of the Earth's evolution.
There is an inconsistency in the partitioning behavior of magnesiowüstite and silicate melt at high pressure on the basis of reports by some authors. According to McFarlane et al. (1994, Geochim. Cosmochim. Acta, 58, 5161-5172), the partition coefficient, KD, between magnesiowüstite and silicate melt for the exchange reaction between Mg and siderophile elements such as Fe, Cr, Mn, and V, are smaller than unity, i.e., these elements are concentrated in the silicate melt, whereas Ni is concentrated in magnesiowüstite (Mg0.93Fe0.07)O at 25 GPa and 2200 °C. On the other hand, according to Agee (1990, Nature, 346, 834-837), KD's for Ni, Fe, Mn, and Cr are greater than unity, i.e., these elements are concentrated in magnesiowüstite (Mg0.32FeO0.68)O at 25-27 GPa and 2075 °C. Such an anomalous partitioning behavior in the FeO-rich portion of the magnesiowüstite solid solution would play an important role in the formation of an FeO-rich core, and would also suggest anomalous melting behavior in the magnesiowüstite solid solution such as a melting minimum. Such anomalous melting behavior, if it does exist, might be related to the existence of a new high-pressure phase in the FeO-rich portion of magnesiowüstite in this pressure range.
In order to test the possible existence of such anormalous melting behavior in FeO-rich magnesiowüstite solid solutions, we have studied the partitioning of Fe, Co, Ni, V, Cr, and Mn between magnesiowüstite and silicate melt at 20 GPa over a wide compositional range of magnesiowüstite solid solutions, through experiments at high pressure and temperature.
The starting materials were equimolar mixtures of magnesiowüstite and olivine solid solutions. Magnesiowustitie was synthesized by mixing about one weight % each of CoO, NiO, V2O3, and MnO, and by heating a stoichiometric mixture of reagents at around 1400 °C under a controlled oxygen fugacity between the QFM and IW buffers. Olivine was also synthesized under similar conditions. The high pressure experiments were conducted at a pressure of 20 GPa using a multianvil press. The quenched samples were polished and examined by optical and scanning electron microscopes. The compositions of the magnesiowüstite and the quenched liquid were measured using the electron microprobe.
We obtained the partition coefficients KD between the silicate melt and a wide range of magnesiowüstite solid solutions from (Mg0.93Fe0.07)O to (Mg0.29Fe0.71)O. We did not observe any significant differences in the partitioning behavior of elements in the MgO- and FeO-rich parts of the magnesiowüstite solid solution. It is interesting to note that the partition coefficients KD of Fe, Co, V, Cr, Mn are smaller than unity whereas that of Ni is greater than unity even for Fe-rich magnesiowüstite compositions. This result, which is not consistent with that of Agee (1990, Nature, 346, 834-837), indicates that there is no evidence for either anormalous melting behavior or for a new phase in FeO-rich compositions at least to 20 GPa. The possibility of a change of the partitioning behavior at higher pressures cannot yet be excluded.
The partition coefficients of transition elements between silicate melt
and mantle minerals, such as olivine, modified spinel, and Mg-perovskite,
determined by the present author are summarized in Figure 3.2-1 together
with the magnesiowüstite - melt partition coefficients determined
in this study. The partitioning behavior of the transition elements between
magnesiowüstite and silicate melt is similar to that between olivine,
modified spinel, and
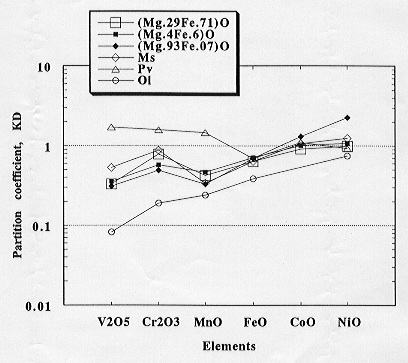 |
Fig. 3.2-1: Element partition coefficients showing the partitioning behaviour of the transition elements between silicate melt and magnesiowüstite (3 compositions), modified spinel (Ms), perovskite (Pv) and olivine (Ol). |
silicate melt. The partitioning behavior of V, Cr, and Mn in these phases and the silicate melt differs significantly from that between perovskite and melt.
The nearly chondritic relative abundances of noble metals in the mantle may be accounted for by the inventory of the late veneer of about 1 wt% accreted after the formation of the Earth's core. However, we can not rule out the possibility that the chondritic relative abundances of these metals in the mantle may be caused by core-mantle equilibrium at very high pressure instead of being due to the late veneer. In order to test this possibility we have conducted preliminary experiments on the partitioning of noble metals, such as Re and Ir, between molten iron and silicate melts in the pressure range 15 to 20 GPa.
We used a double capsule consisting of a rhenium outer capsule and a magnesia inner capsule. We used a mixture of 30 wt% Fe, 35 wt% Re, and 35 wt% Ir as a metal component of the starting material, together with olivine (Fo60Fa40) as the silicate component. Equal volumes of the metal and silicate components were enclosed in the double capsule as separate layers with the silicate layer situated above the metal layer. After the multianvil experiments, we observed a large metal pool surrounded by quenched silicate liquid within the magnesia capsule. This texture consisting of relatively distinct pools of metal and silicate melt, made it possible to analyze the chemical compositions of molten iron, silicate melt, and magnesiowüstite using the electron microprobe. The analysis of Re and Ir in the run products is currently in progress to obtain the partition coefficients of these elements at very high pressure.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page