

As part of our ongoing research into the stability of platinum group metal-bearing oxide phases (such as MgRh23+O4 and geologically important solid solutions containing this endmember), we conducted experiments to determine the mixing behaviour of MgRh23+O4-MgAl2O4 solid solutions. In order to overcome the limitations imposed by sluggish reaction kinetics at lower temperatures, we constructed diffusion couples between MgAl2O4 single crystals and disks of Rh metal. The samples were reacted at 1117 °C in mixtures of oxygen and Ar for 14 days, which resulted in the metal and spinel being sintered together tightly. Examination with microbeam techniques of sections cut perpendicular to the metal-spinel interface showed the presence of ß-Rh2O3 in all samples, indicating that the samples were not in equilibrium with the gas mixtures, but were likely buffered by ß-Rh2O3 instead.
We expected to find the same equilibrium spinel composition in all samples, given that they were all likely buffered by the same reaction. No reaction was observed to have occurred between metal and spinel at the resolution of the electron microprobe. However, after repolishing the samples followed by examination using a scanning electron microscope, we found that a layer of Mg(Rh,Al)2O4 spinel had formed as a separate phase at the interface between metal and spinel. As expected, the composition of the spinel layer was the same in all samples.
The composition of the Mg(Rh,Al)2O4 spinel in
equilibrium with Rh and ß-Rh2O3
may be used to constrain the interaction coefficient of MgRh23+O4
in MgRh23+O4 - MgAl2O4
solid solutions. In Fig. 3.3-5 we plot the chemical potential of oxygen
as a function of the Rh/(Rh + Mg) ratio at 1100 °C in the system Mg-Rh-O.
The most important feature of the diagram is the considerable extent of
solid solution between MgRh23+O4 and Mg2Rh4+O4.
In fact, pure MgRh23+O4 is only found
in equilibrium with ß-Rh2O3.
At the oxygen fugacities below the Rh-ß-Rh2O3
buffer, MgRh2O4 breaks down according to the reaction
2 MgRh23+O4 = Mg2Rh4+O4
+ 3 Rh + 2 O2. In our experiments the rhodate spinel is in equilibrium
with both Rh and ß-Rh2O3,
corresponding to point A in Fig. 3.3-5. Assuming a regular solution model
and that the activity of Mg2RhO4 in our alumina-bearing
system is the same as in an alumina-free system, we calculate a large positive
deviation from ideality for the MgRh23+O4-MgAl2O4
solid solution. Such a large positive deviation may be partly responsible
for the reluctance of rhodium to diffuse into MgAl2O4.
However additional measurements in which the compositional dependence of
the interaction coefficient of MgRh2O4 must be evaluated
are needed.
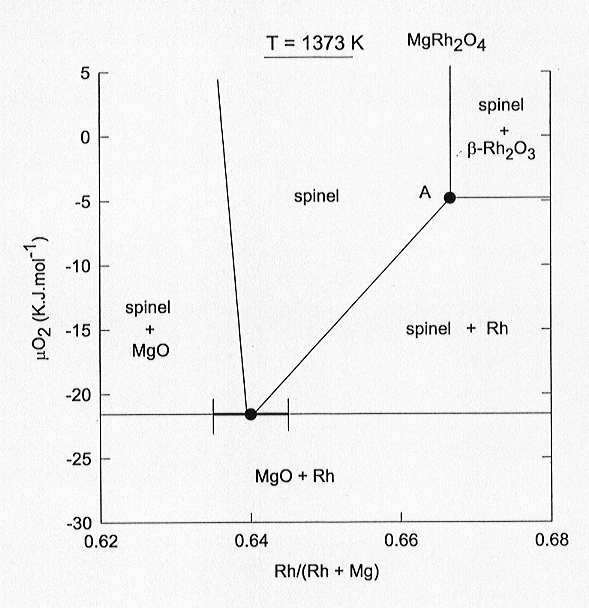 |
Fig. 3.3-5: Chemical potential of oxygen as a function of the Rh/(Rh + Mg) ratio in the system Mg-Rh-O at 1100 °C. At point "A" spinel coexisting with Rh and ß-Rh2O3 contains virtually no Mg2Rh4+O4 in solid solution. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page